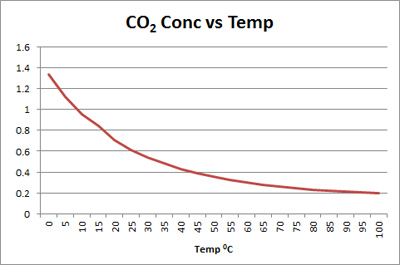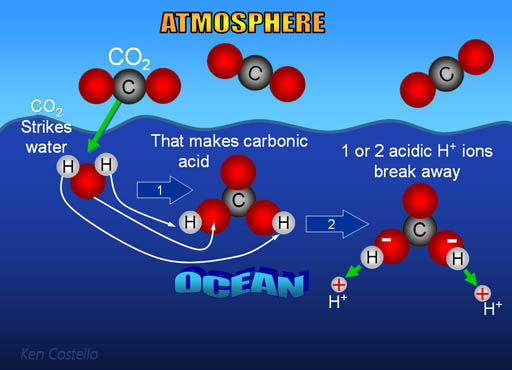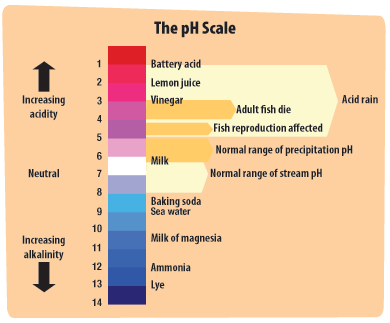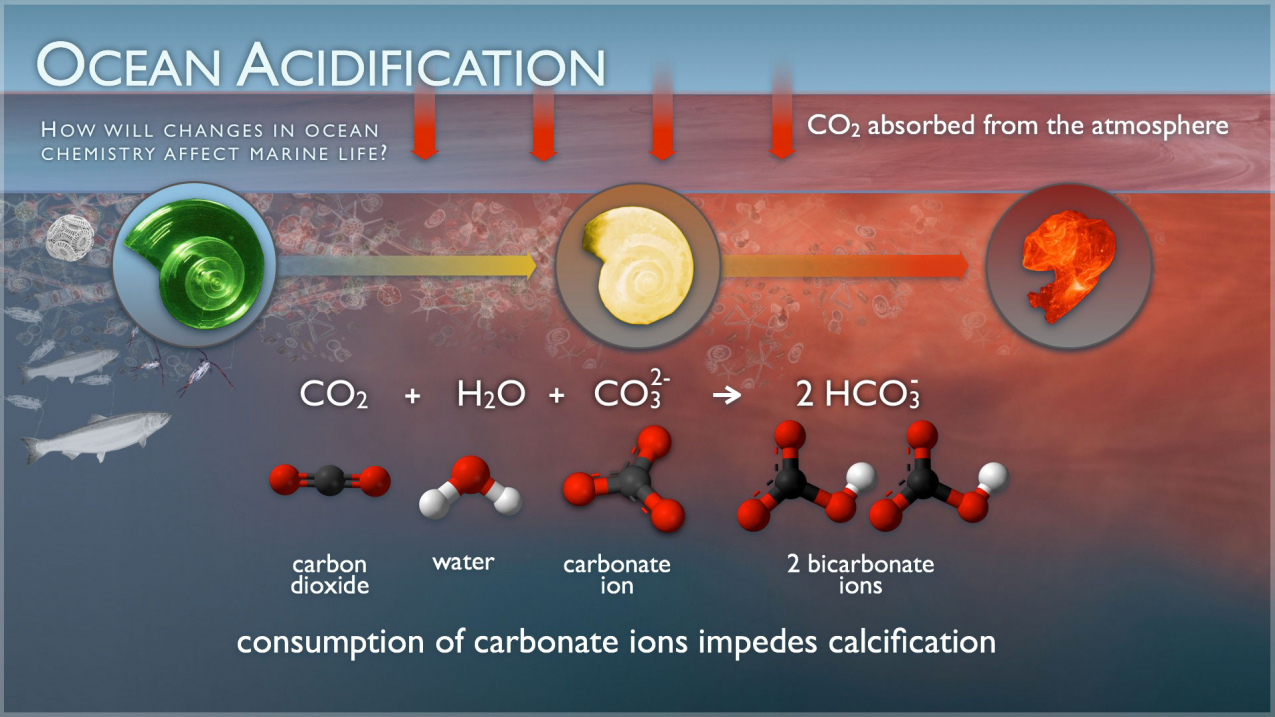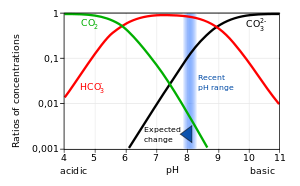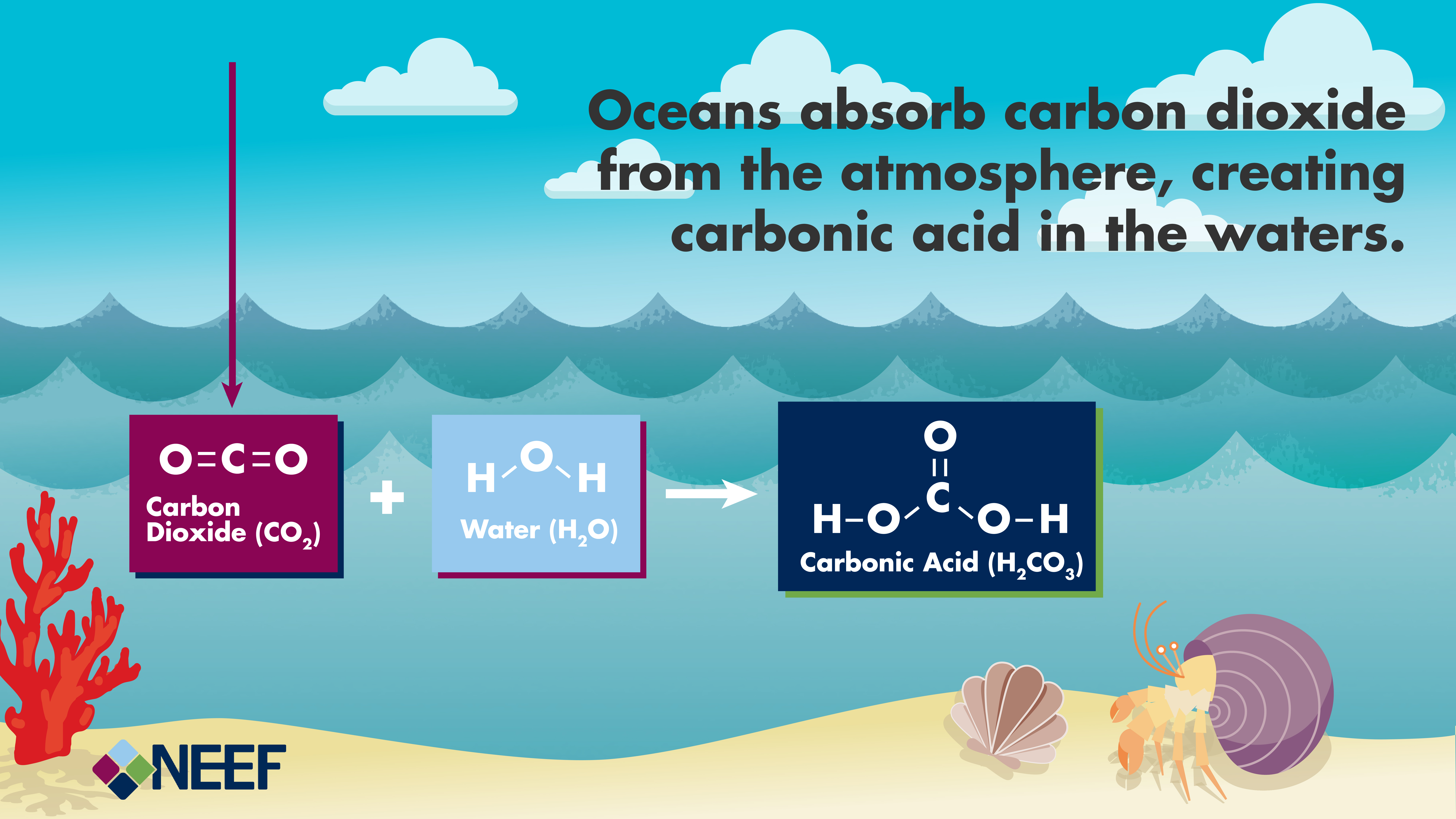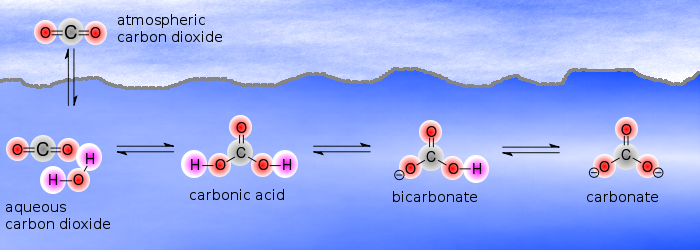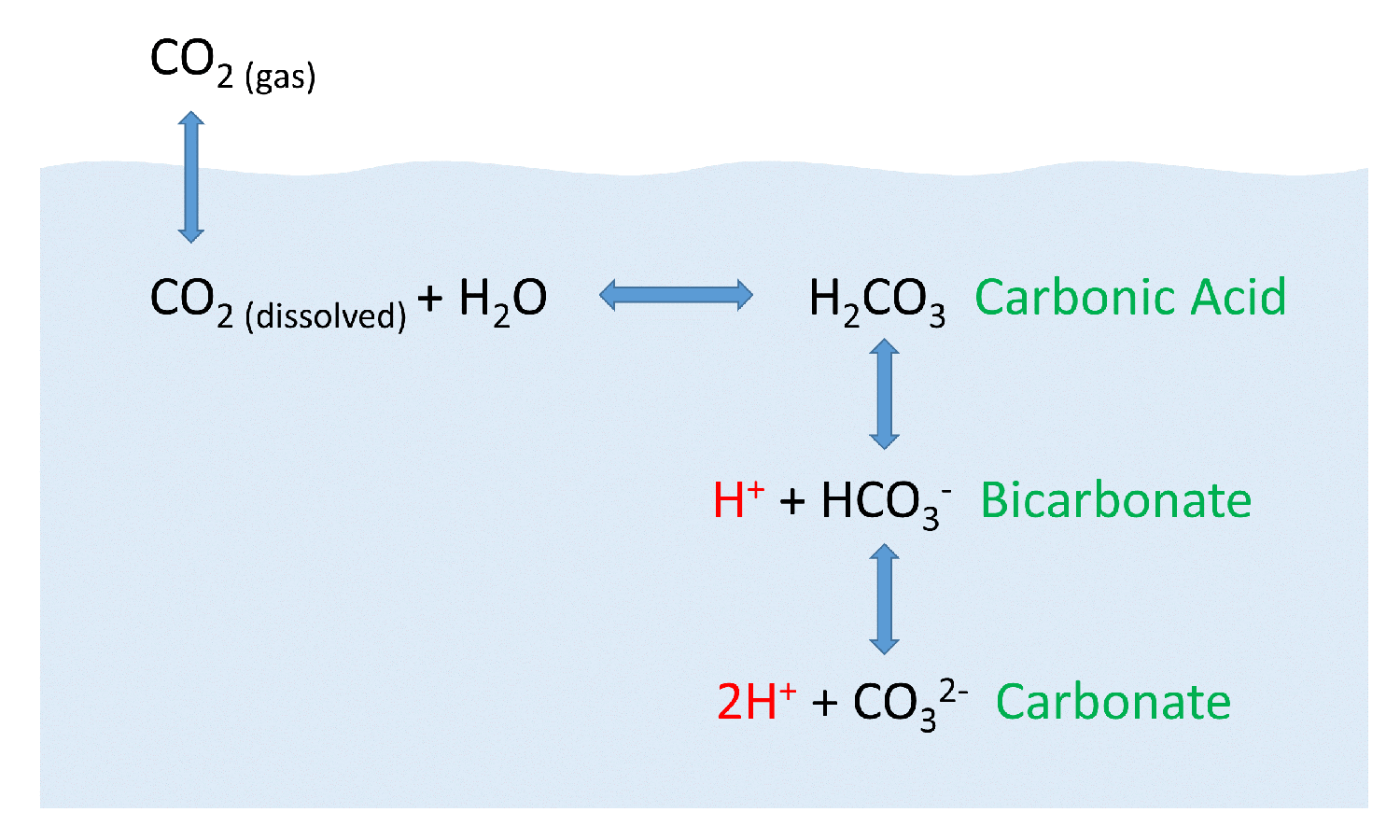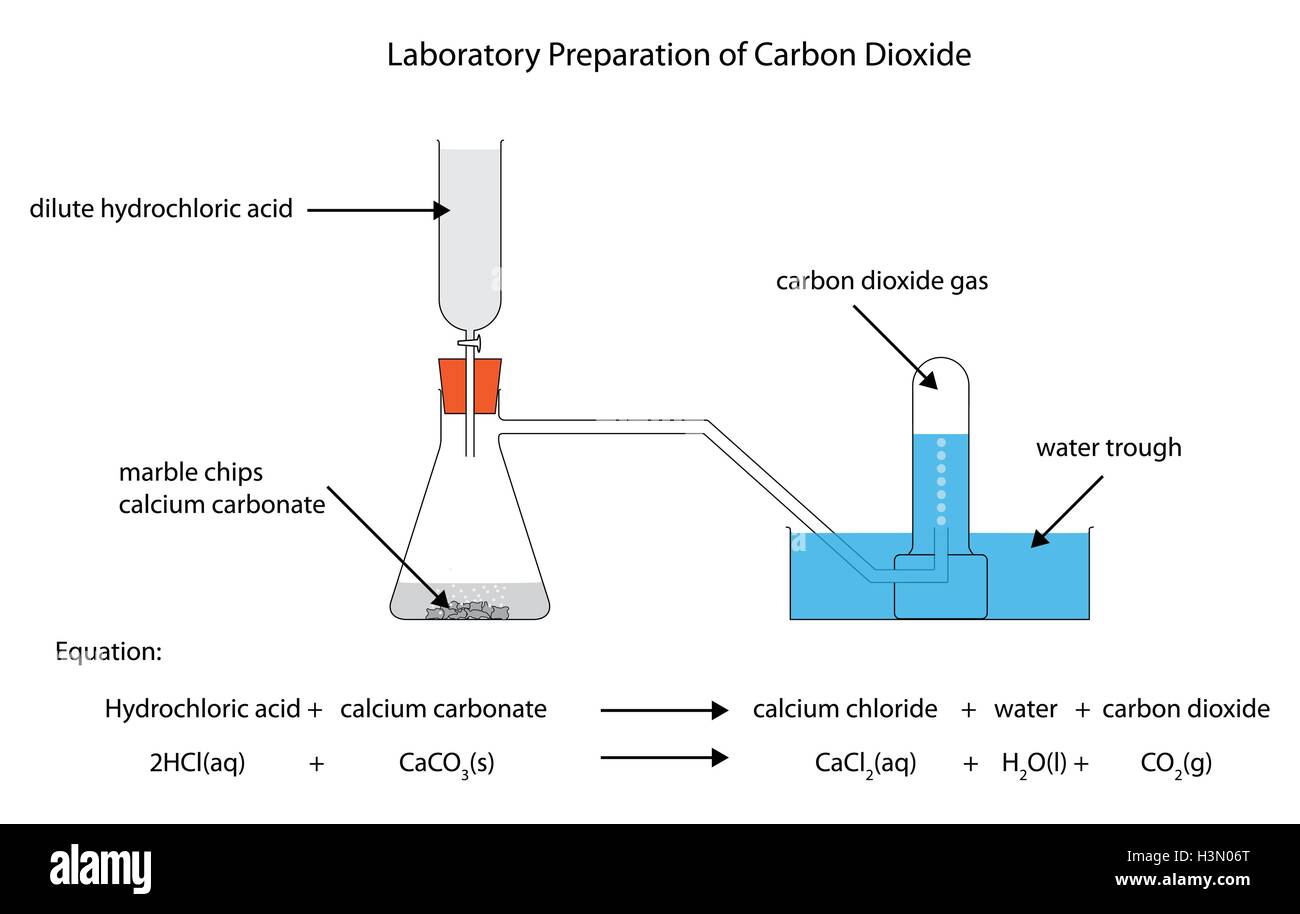
Diagram of the laboratory preparation of carbon dioxide from hydrochloric acid and calcium carbonate marble chips Stock Vector Image & Art - Alamy

Catalytic conversion of carbon dioxide to carboxylic acid derivatives - Liu - 2015 - Greenhouse Gases: Science and Technology - Wiley Online Library
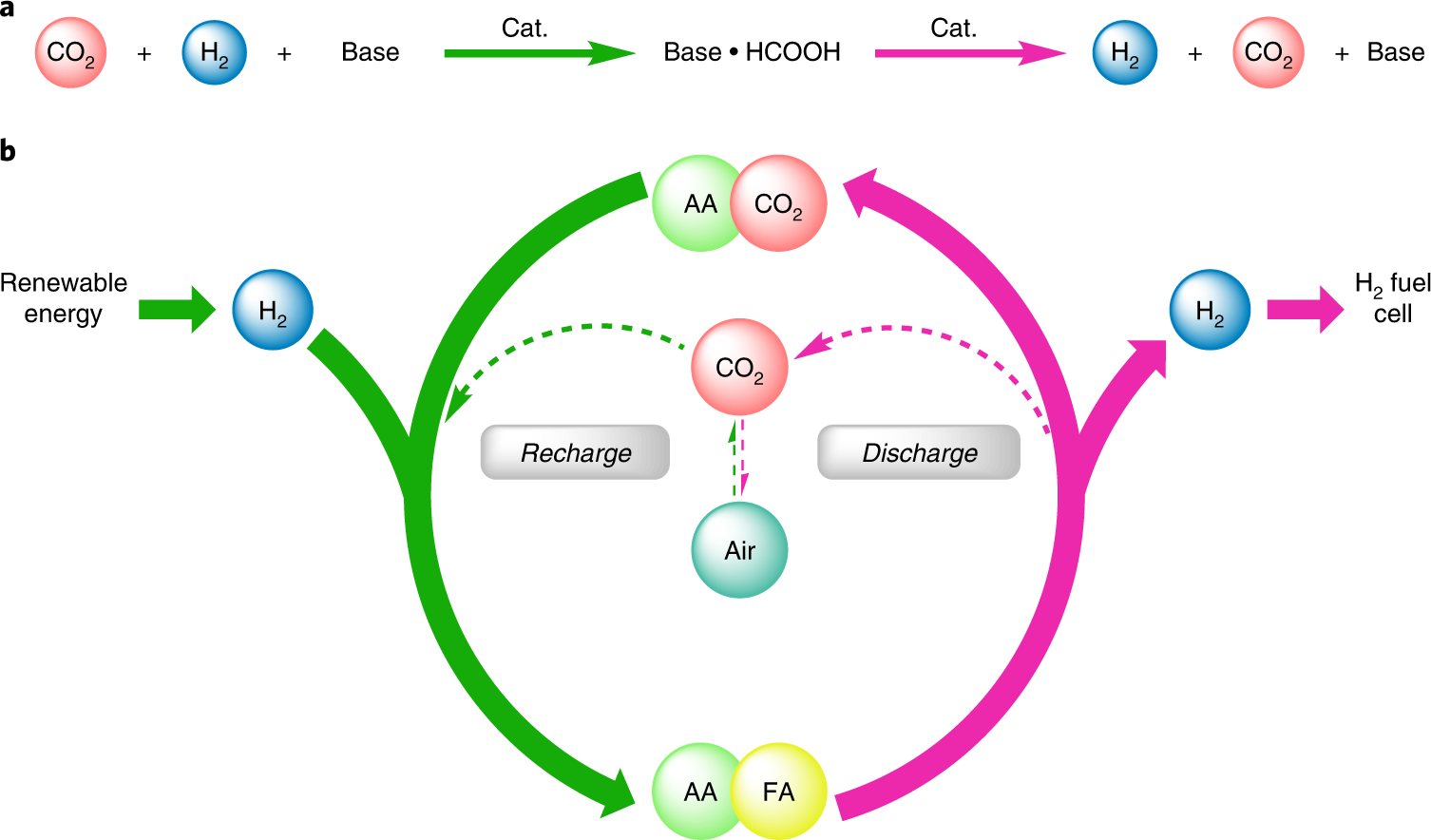
Reversible hydrogenation of carbon dioxide to formic acid using a Mn-pincer complex in the presence of lysine | Nature Energy

Bioproduction of acetic acid from carbon dioxide as single substrate and zero valent iron (ZVI) by clostridia - ScienceDirect