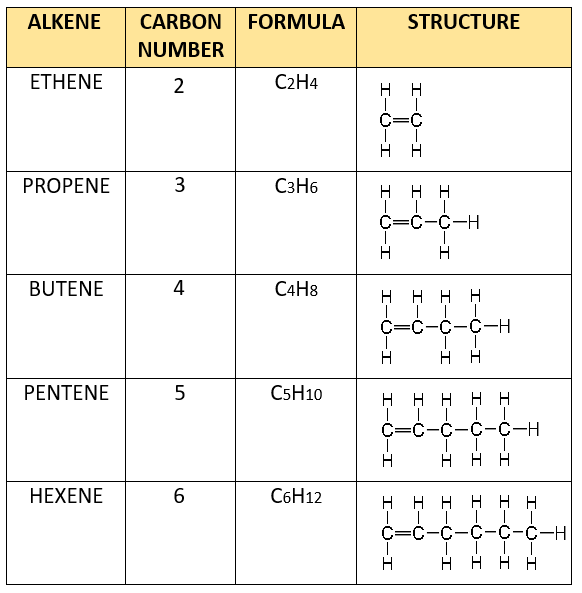
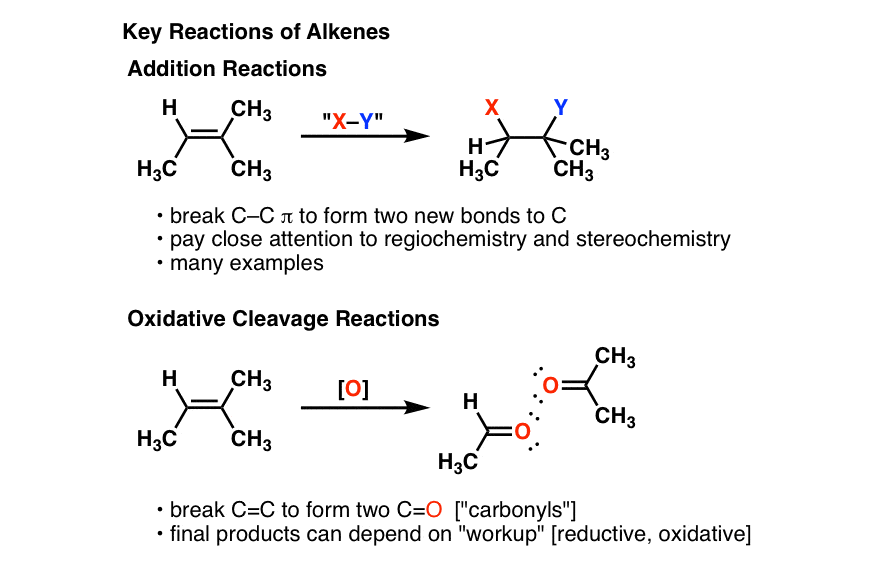
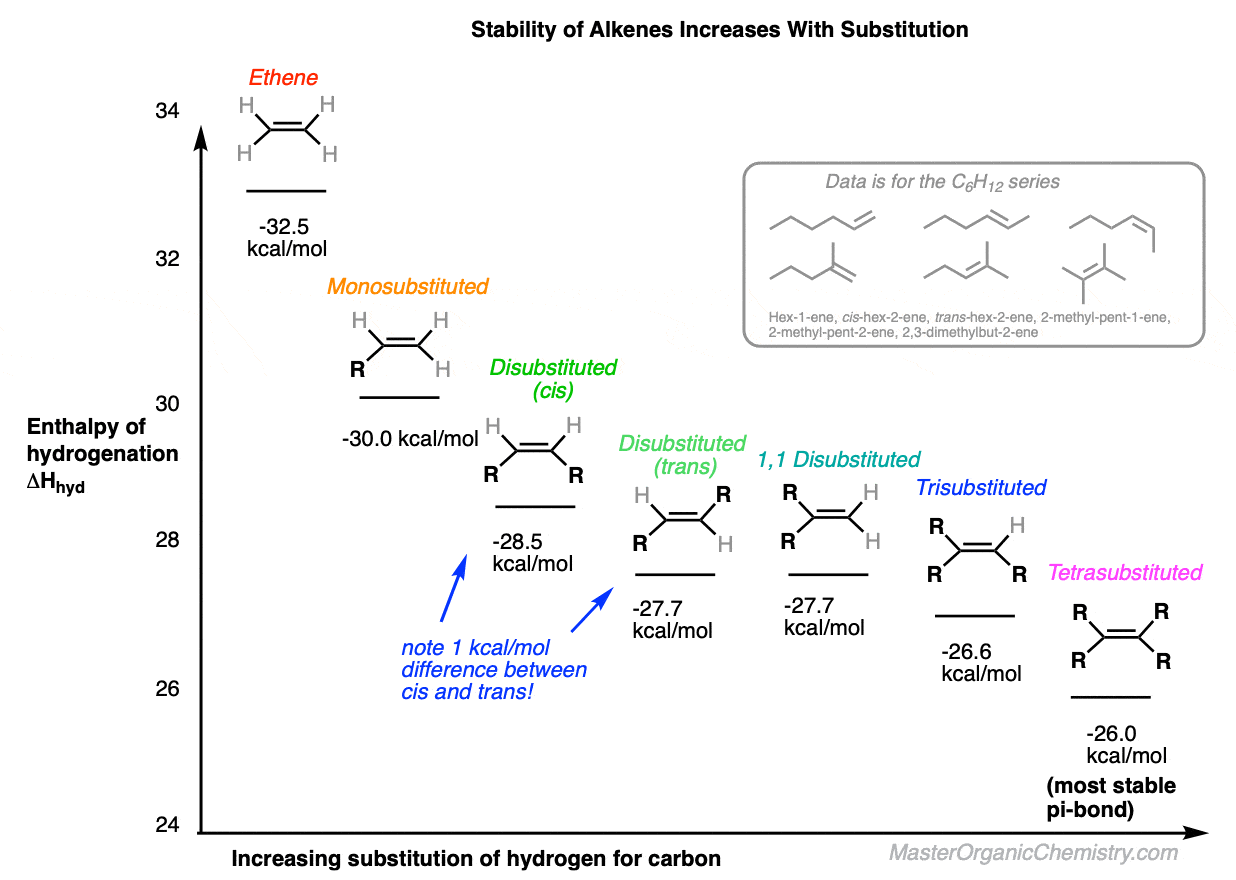
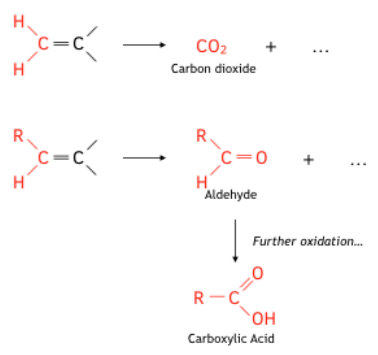
Alkenes - unsaturated hydrocarbons, molecular structure, chemical properties, uses, reactions bromine hydrogen, ethene C2H4, propene C3H6, butene C4H8 gcse chemistry revision notes igcse revising KS4 science
![Draw structures of alkenes (Full structural formula) [Online Lesson] – O Level Secondary Chemistry Tuition Draw structures of alkenes (Full structural formula) [Online Lesson] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2022/01/picture8.jpg?w=1024)
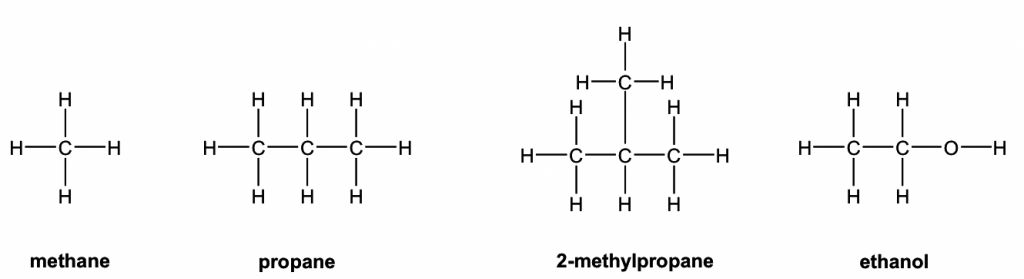
Draw structures of alkenes (Full structural formula) [Online Lesson] – O Level Secondary Chemistry Tuition
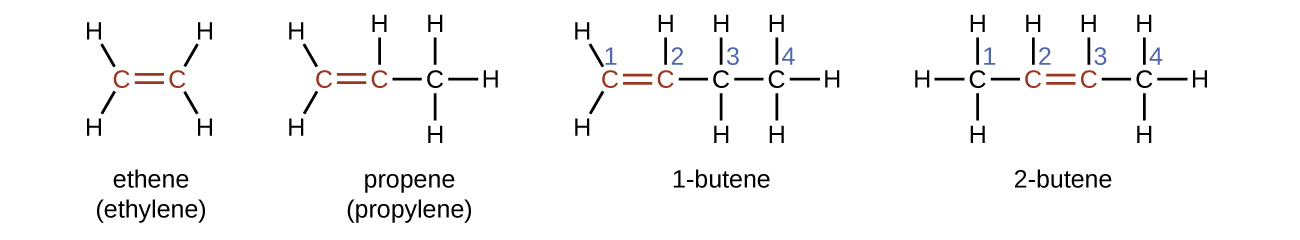
M12Q3: Alkenes: Naming, Geometric Isomers, Intermolecular Forces and Bond Properties; Optical Isomers – Chem 103/104 Resource Book

Alkenes - unsaturated hydrocarbons, molecular structure, chemical properties, uses, reactions bromine hydrogen, ethene C2H4, propene C3H6, butene C4H8 gcse chemistry revision notes igcse revising KS4 science

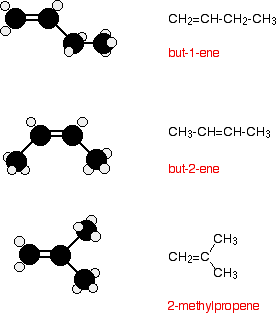
3.7 draw displayed formulae for alkenes with up to four carbon atoms in a molecule, and name the straight-chain isomers – igcse2016

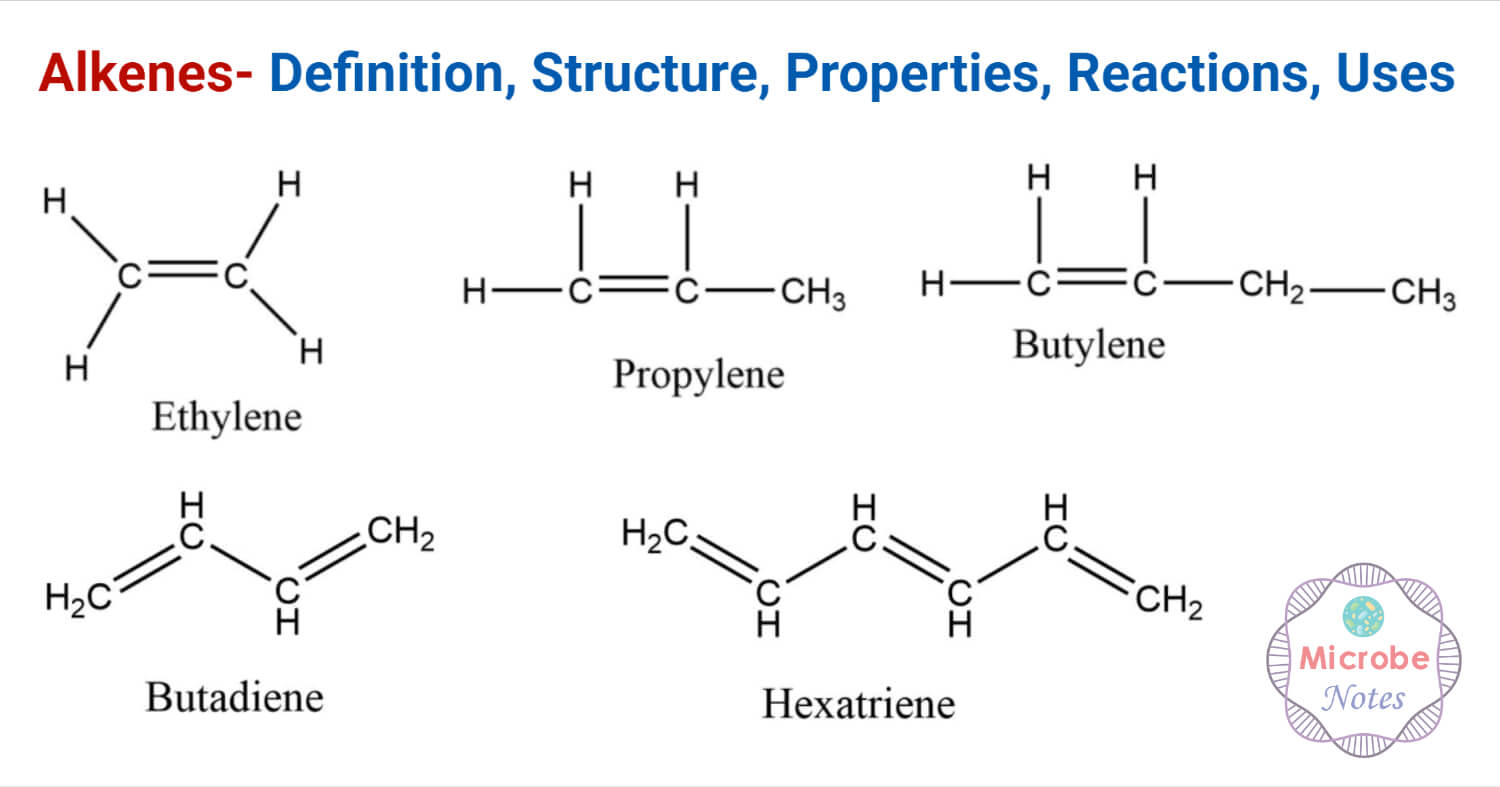
Alkenes - Introduction (1.10.1) | Edexcel International A Level Chemistry Revision Notes 2017 | Save My Exams