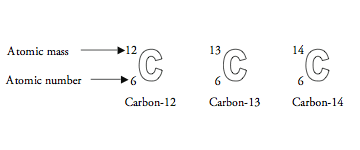
Carbon-13 Carbon-12 Atomic nucleus Carbon-14 Atomic mass, others, carbon, isotope, proton png | PNGWing

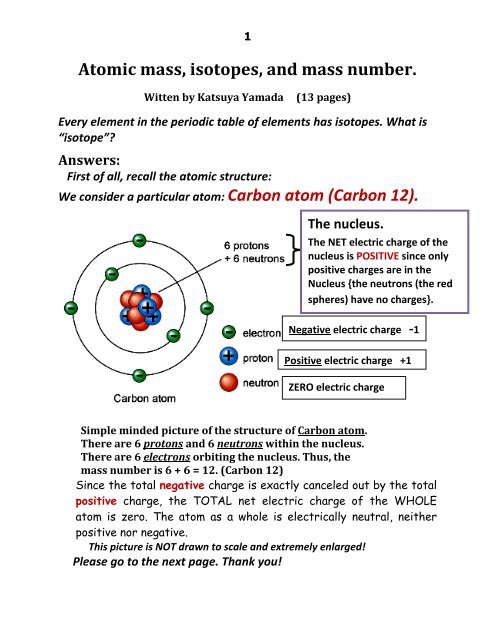
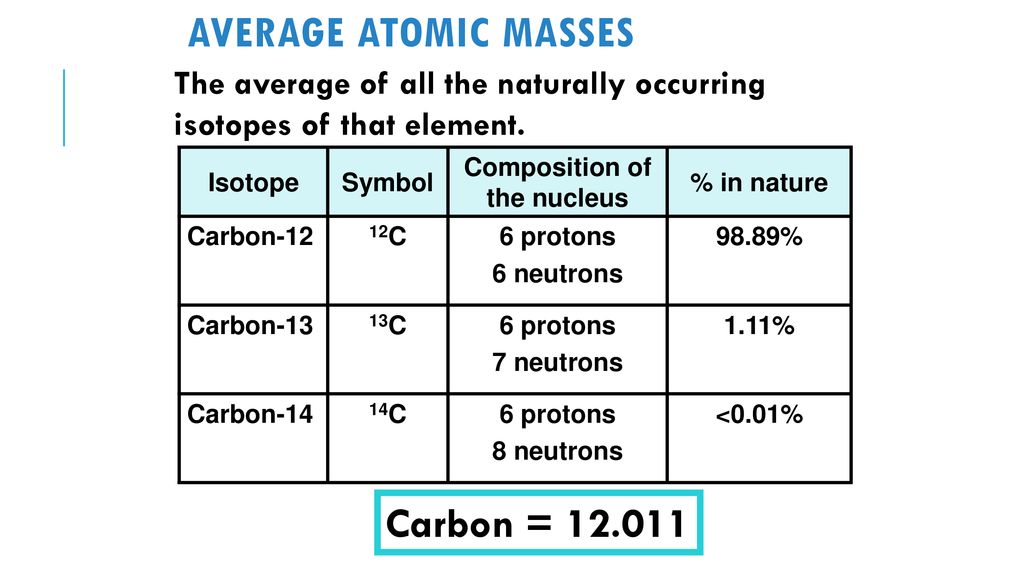
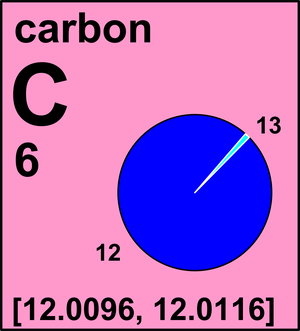
Carbon occur in nature as a mixture of `C-12` and `C-13`. Average atomic mass of carbon is `12.011 - YouTube

Katie Mummah on Twitter: "So the atomic mass has to take into account these isotopes. Each of the individual atomic masses is weighted by it's natural abundance to get the atomic weight

SOLVED:The two most abundant naturally occurring isotopes of carbon are carbon- 12(98.90 %, 12.000 amu) and carbon-13 (1.10 %, 13.003 amu). From these abundances, calculate the atomic weight of carbon and compare
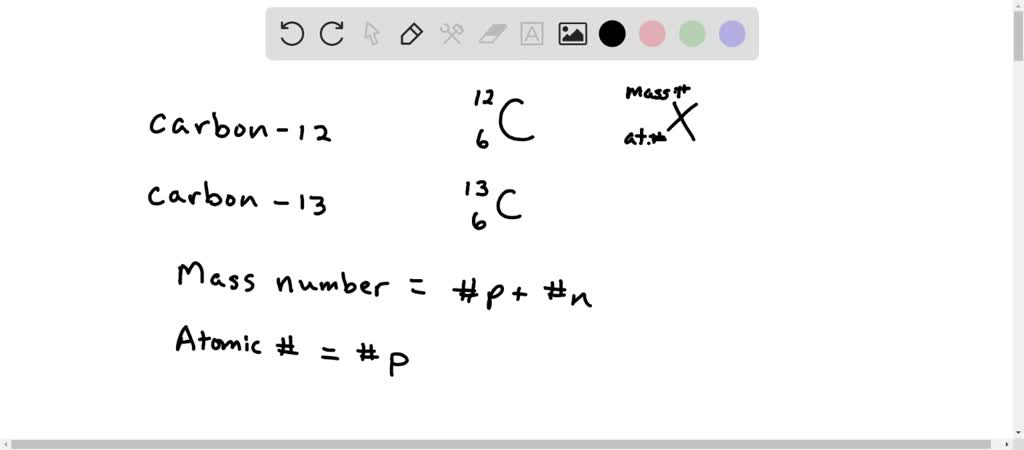
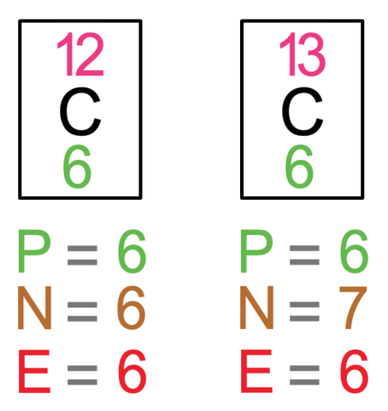
SOLVED: List the mass number and atomic number of carbon-12 and carbon-13, respectively. a. The mass number and atomic number of carbon-13 is 13 and 6, while that of carbon-12 is 12

The common isotopes of carbon are ^12C and ^13C . The average mass of carbon is 12.01115 amu. What is the abundance of ^13C isotope ?.

SOLVED: Calculate the average atomic mass of carbon if 98.90% of the atoms are C-12 (12.000000 amu) and 1.100% are C-13 atoms (13.003354 amu).
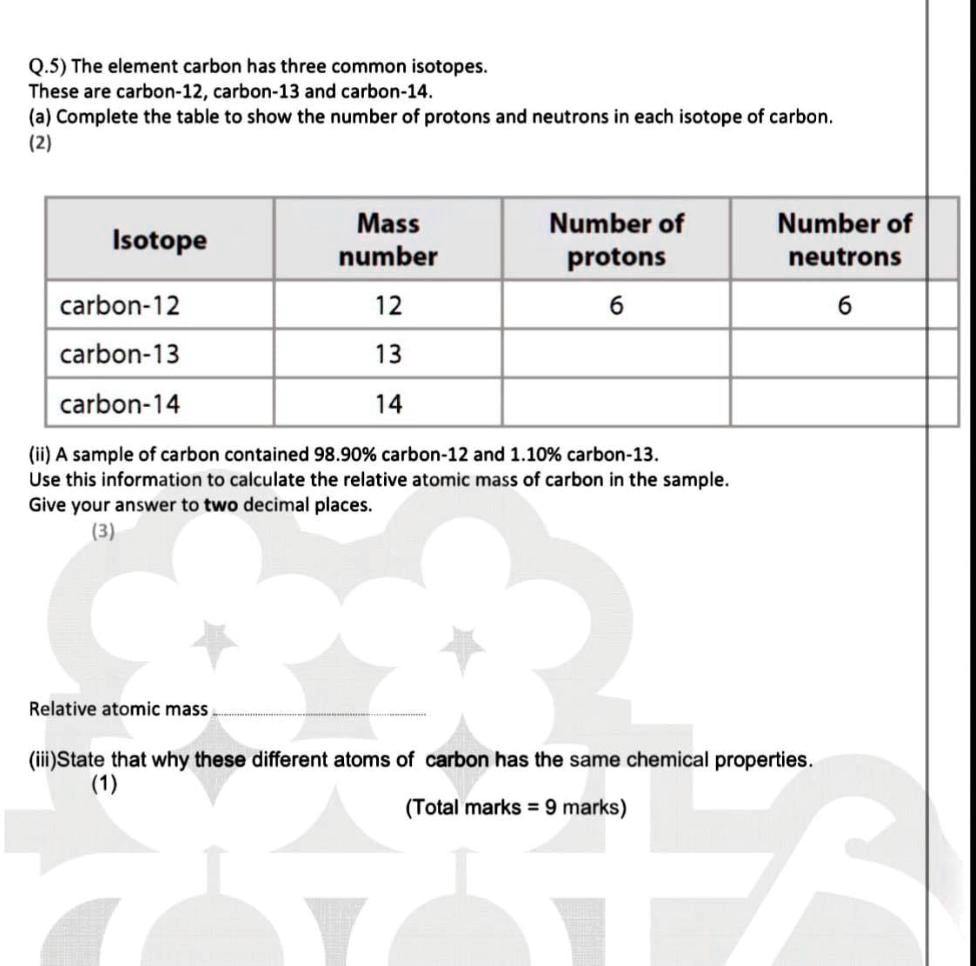
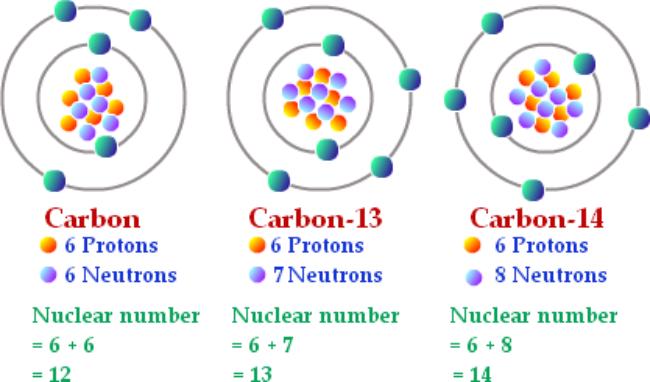
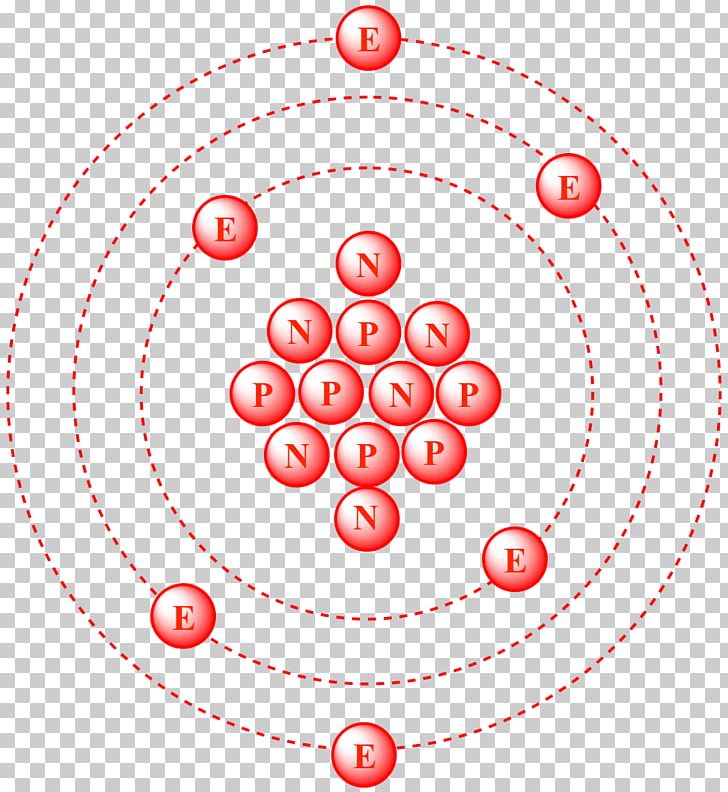
SOLVED: Q.5) The element carbon has three common isotopes: These are carbon-12, carbon-13 and carbon-14 (a) Complete the table to show the number of protons and neutrons in each isotope of carbon: (