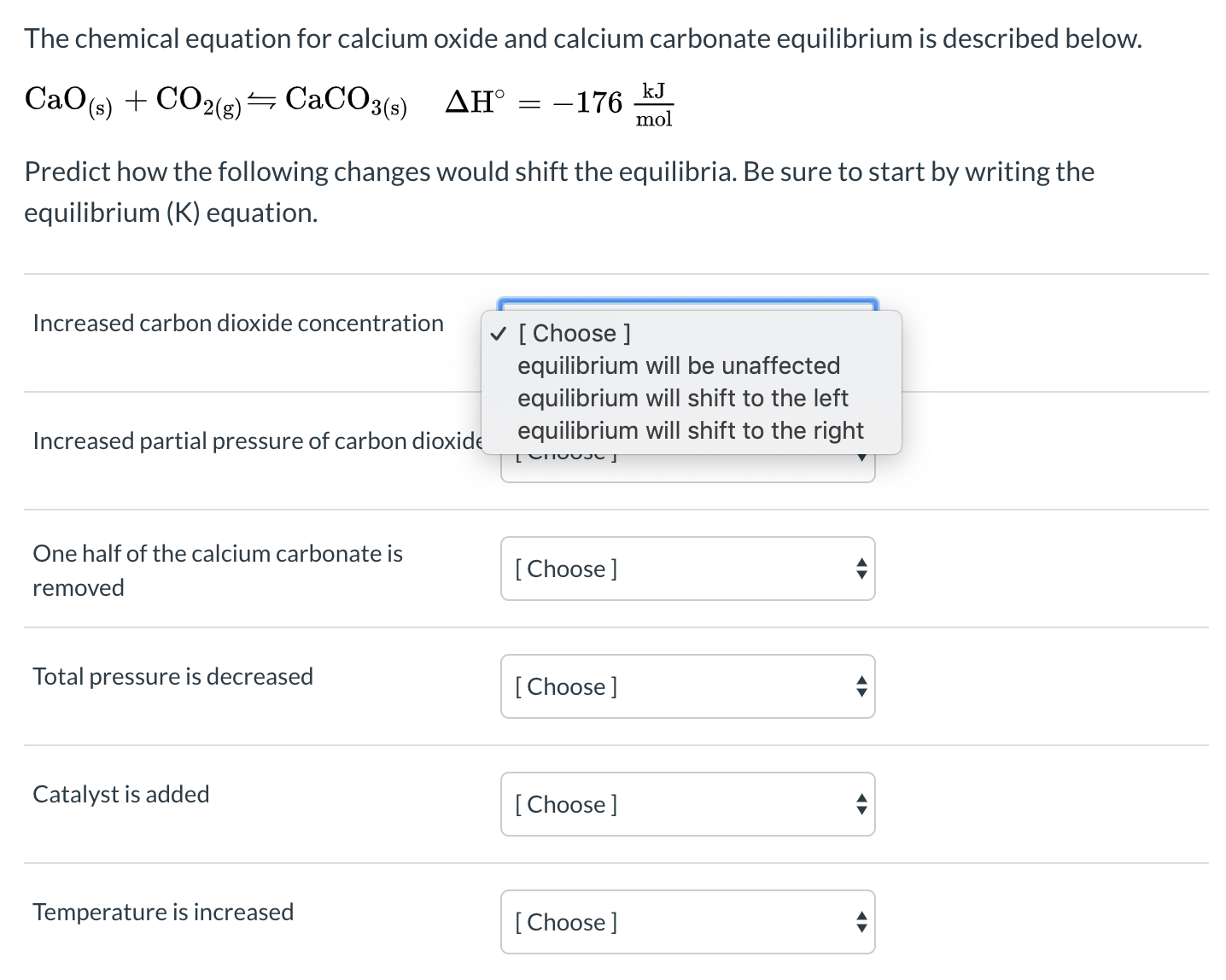
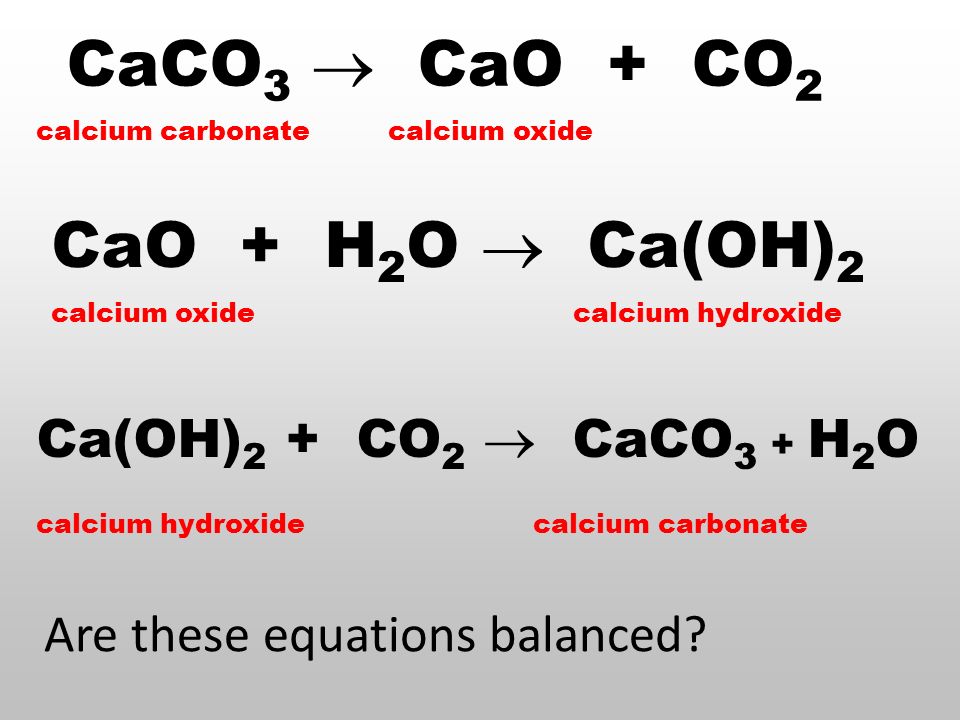
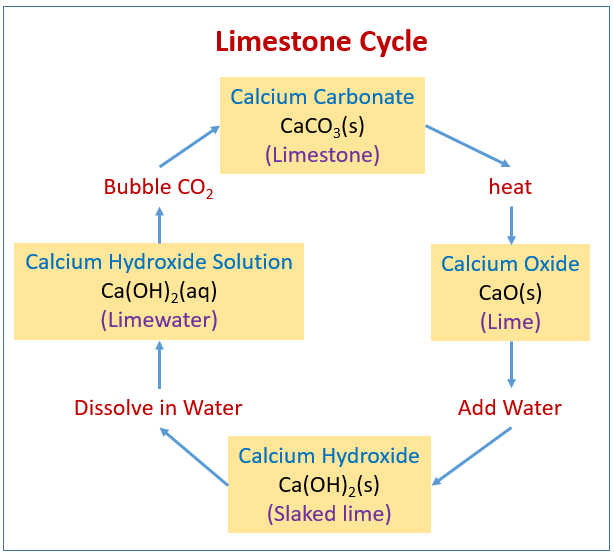
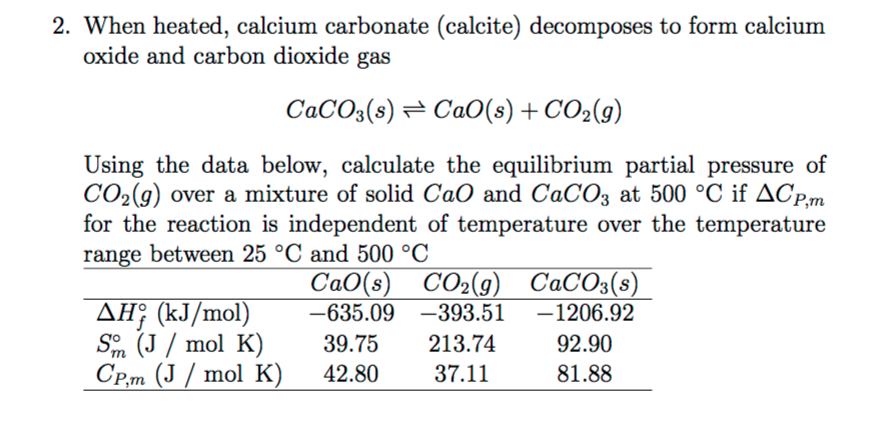
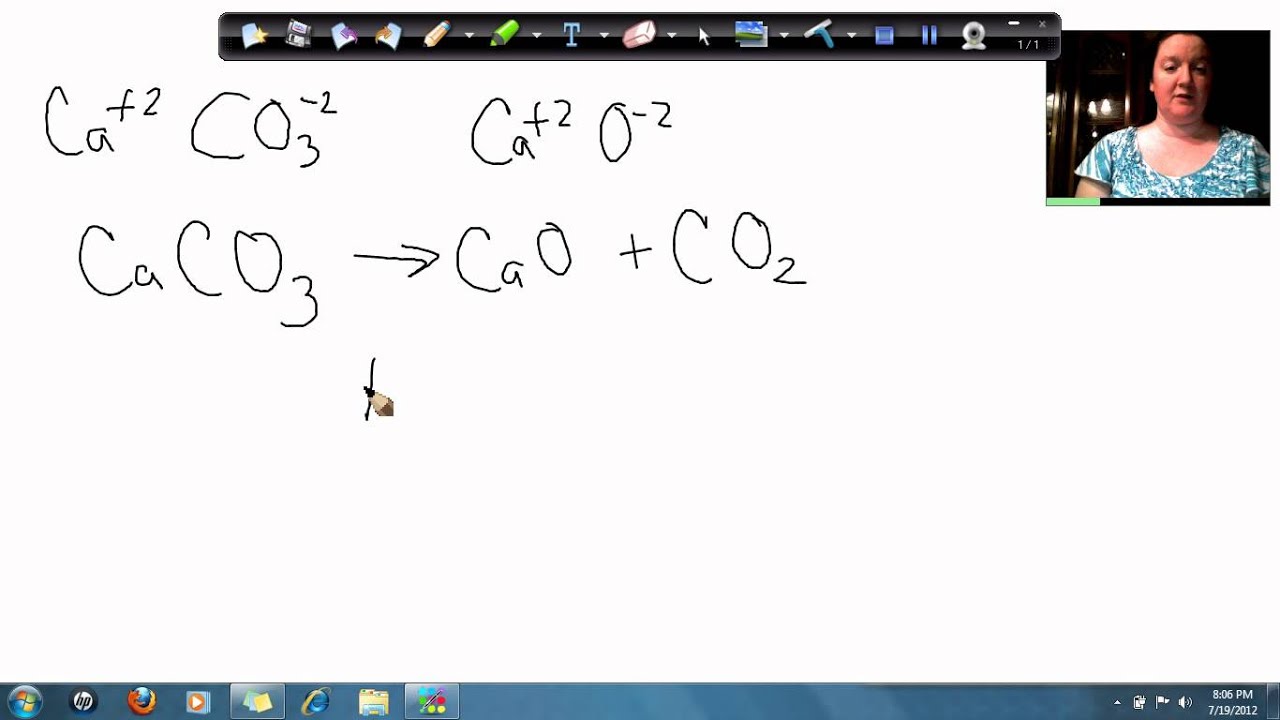50 g of an impure calcium carbonate sample decomposes on heating to give carbon dioxide and 22.4 g calcium oxide. The percentage purity of calcium carbonate in the sample is:

Characterization of Calcium Carbonate, Calcium Oxide, and Calcium Hydroxide as Starting Point to the Improvement of Lime for Their Use in Construction | Journal of Materials in Civil Engineering | Vol 21, No 11
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate - Energy & Environmental Science (RSC Publishing)

Calcium Carbonate the Result of the Reaction of Calcium Oxide with Carbon Dioxide. Being Prepared in Petri Dish Stock Image - Image of ammonium, dioxide: 204865573

Calcium extraction from steelmaking slag and production of precipitated calcium carbonate from calcium oxide for carbon dioxide fixation - ScienceDirect

Difference Between Calcium and Calcium Carbonate | Chemical Properties, Occurrence, Uses, Differences

inorganic chemistry - Why does calcium carbonate decompose into calcium oxide? - Chemistry Stack Exchange

Question Video: Identifying the Chemical Equation- with State Symbols- That Corresponds to a Chemical Statement | Nagwa

Flowchart for the process of obtaining calcium carbonate and calcium... | Download Scientific Diagram
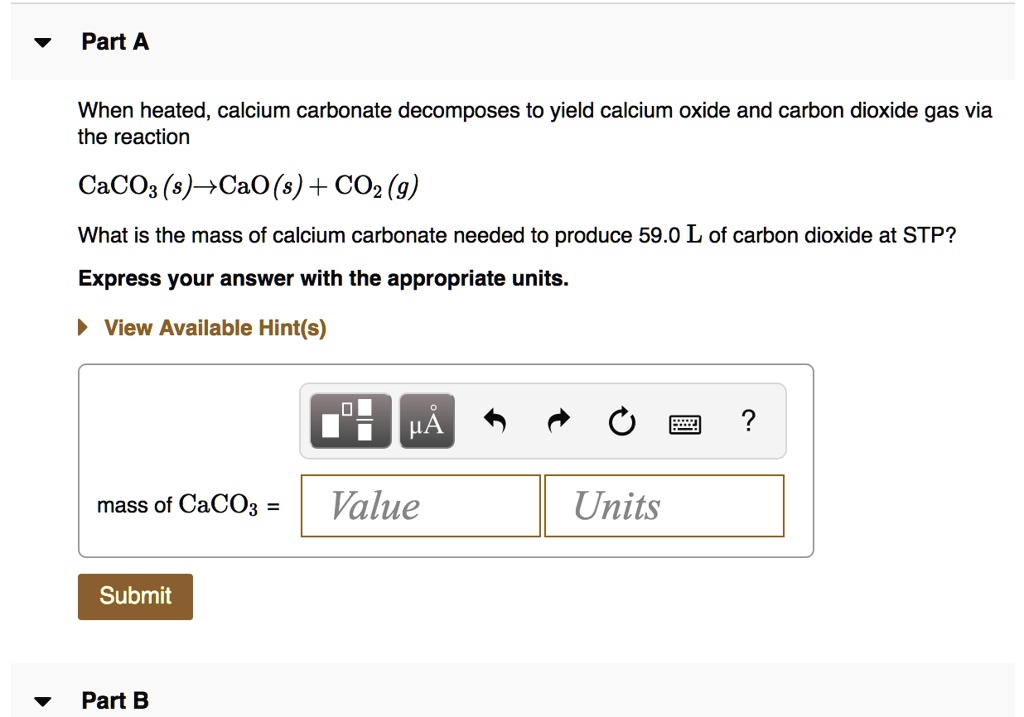
SOLVED: Part A When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction CaCOs (s)-CaO (s) + CO2 (g) What is the mass of calcium carbonate

Characterization of Calcium Carbonate, Calcium Oxide, and Calcium Hydroxide as Starting Point to the Improvement of Lime for Their Use in Construction | Journal of Materials in Civil Engineering | Vol 21, No 11

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride

Calcium carbonate decomposes, on heating , to form calcium oxide and carbon dioxide. When 10 g of - YouTube