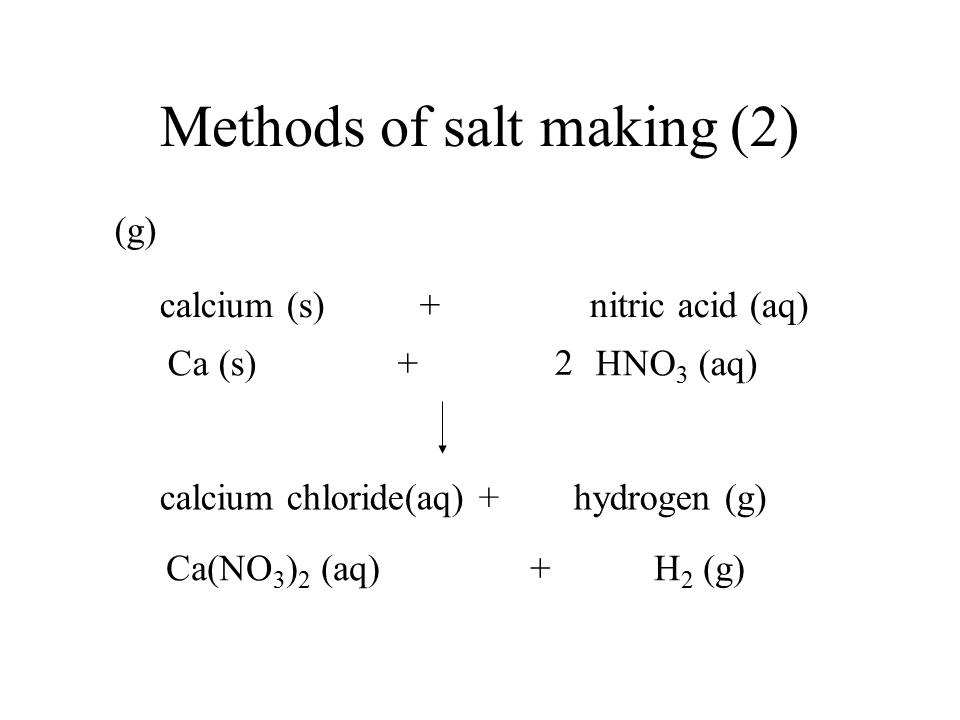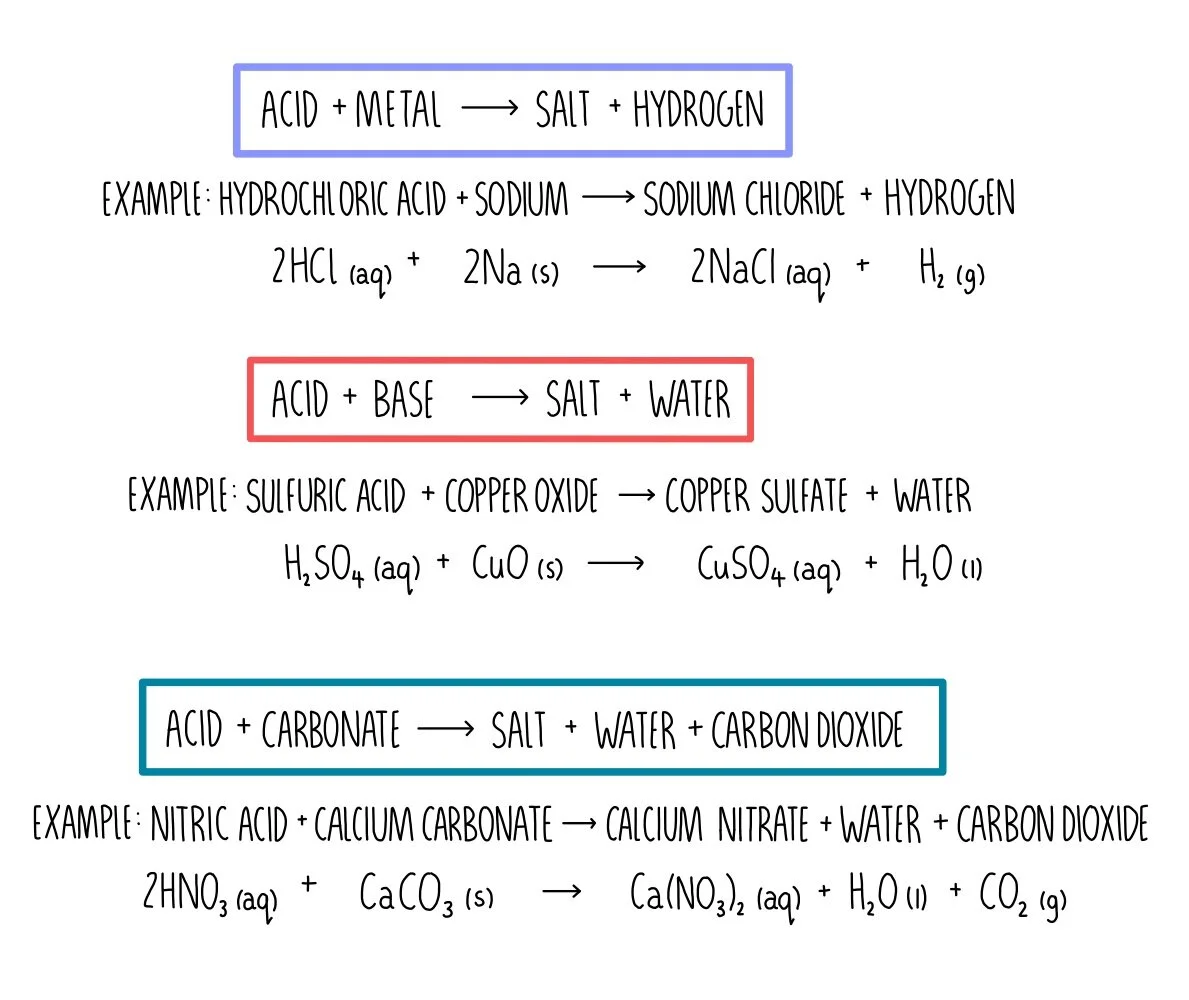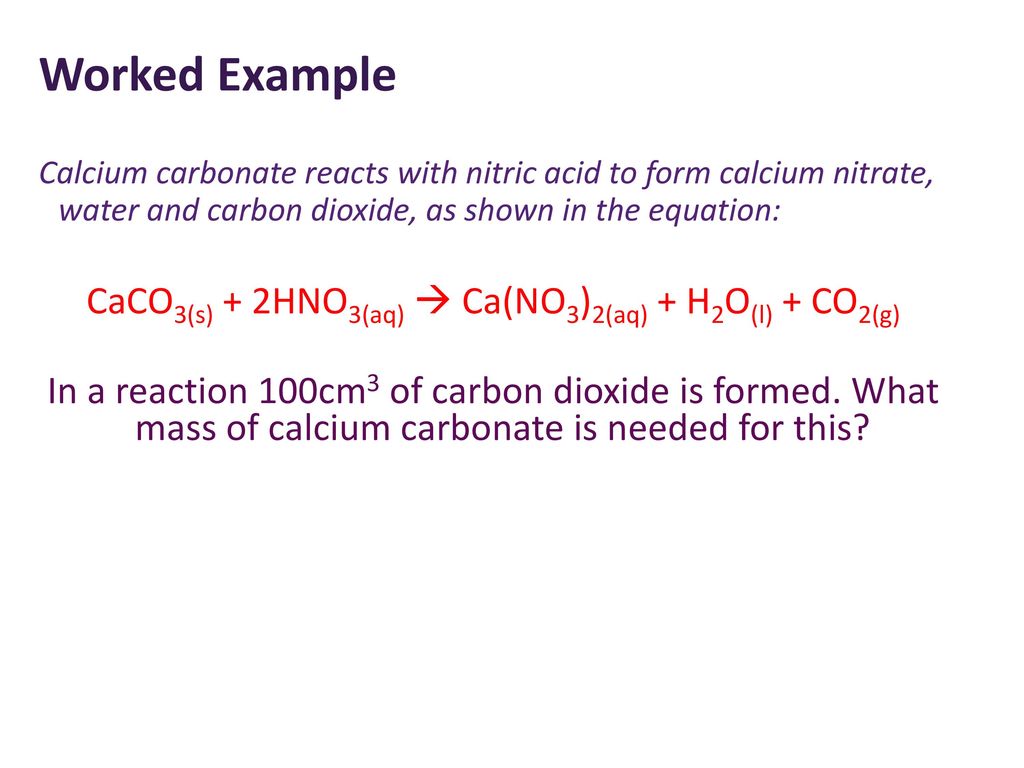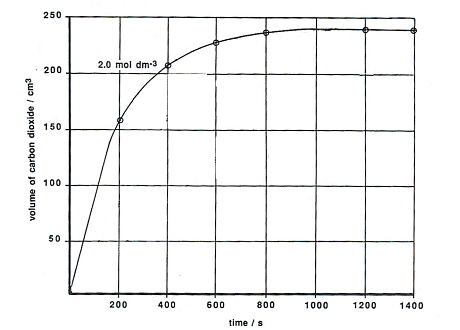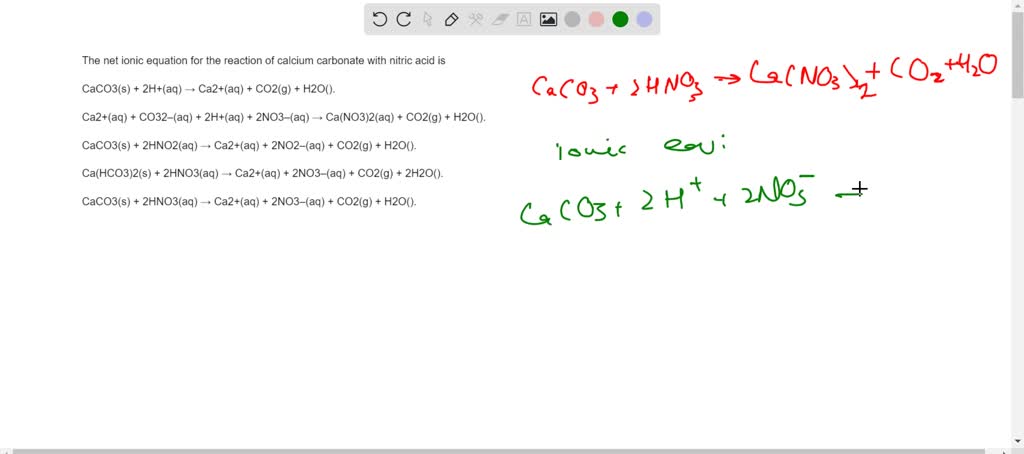
SOLVED: The net ionic equation for the reaction of calcium carbonate with nitric acid is CaCO3(s) + 2H+(aq) → Ca2+(aq) + CO2(g) + H2O(). Ca2+(aq) + CO32–(aq) + 2H+(aq) + 2NO3–(aq) →
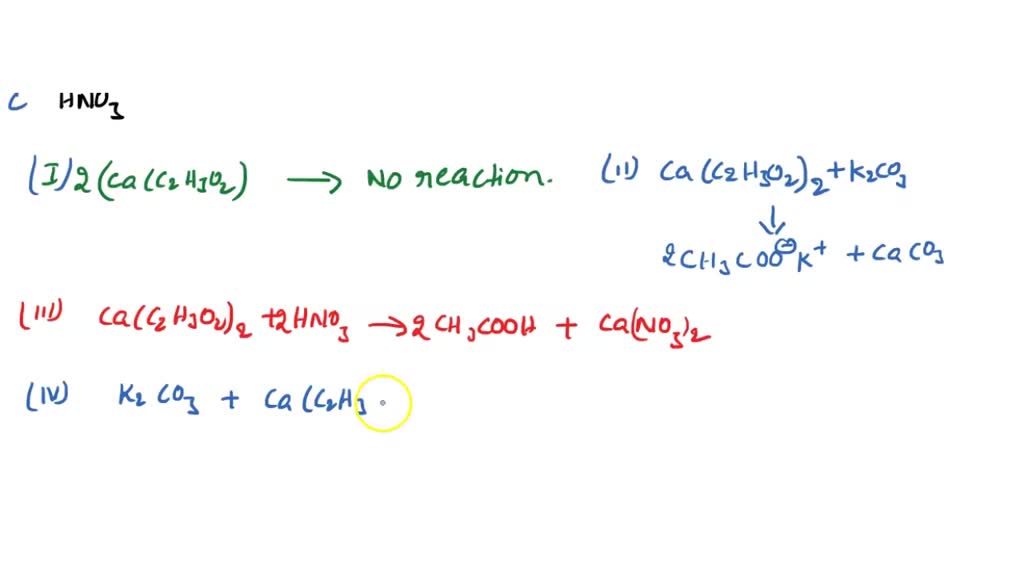
SOLVED: You are given solutions of calcium acetate, potassium carbonate and nitric acid. Complete a data table similar to those in the background. Write equations for all reactions that occur Ca(C2H3O2)2 K2CO3

OneClass: Write a net ionic equation for the reaction that occurs when calcium carbonate (s) and exce...

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

How would you write the name of the following compounds ◦ Zn(OH) 2 ◦ NaOH ◦ HCl ◦ Mg(NO 3 ) 2 What does an acid do to red litmus? What does an acid to. - ppt download