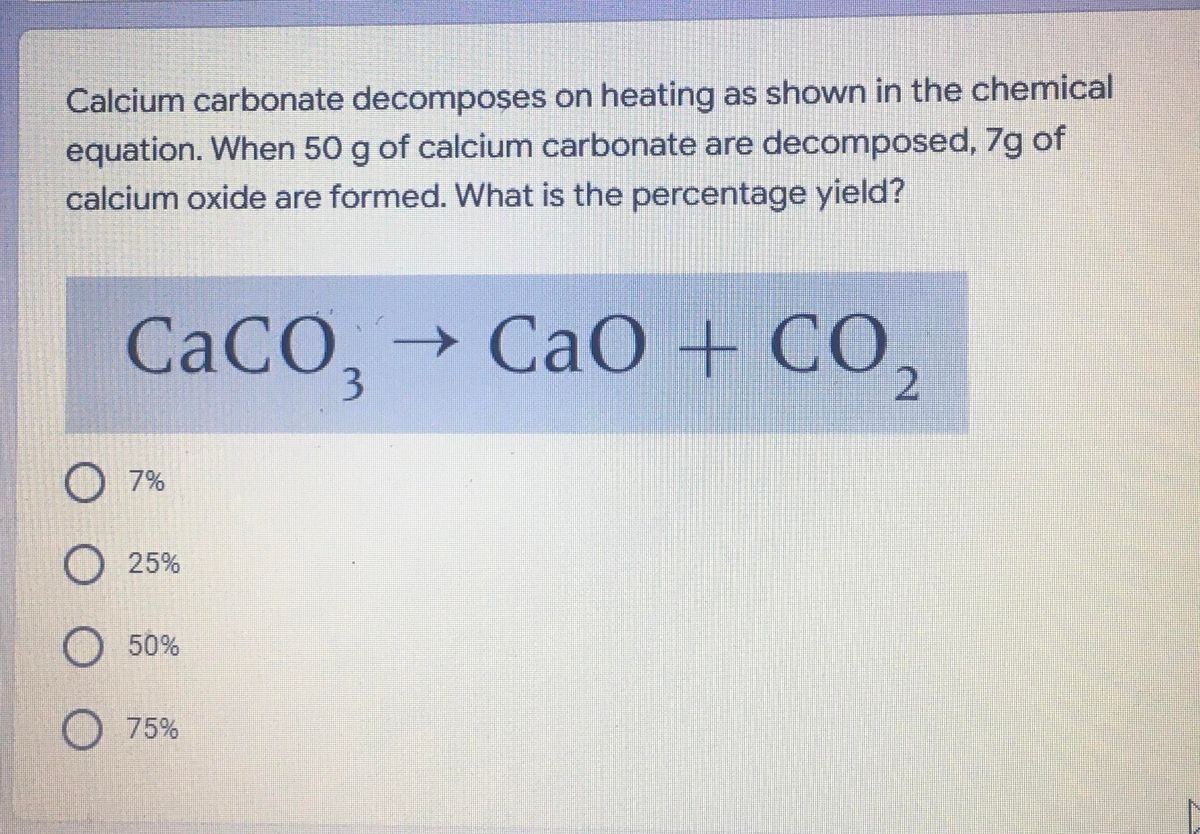
Question Video: Identifying the Chemical Equation- with State Symbols- That Corresponds to a Chemical Statement | Nagwa

When calcium carbonate (CaCO3) is heated, it decomposes to form calcium oxide (CaO) and carbon - Brainly.com

OneClass: Consider the decomposition of calcium carbonate: CaCO_3(s) Equilibrium CaO(s) + CO_2(g) del...

Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction - ScienceDirect

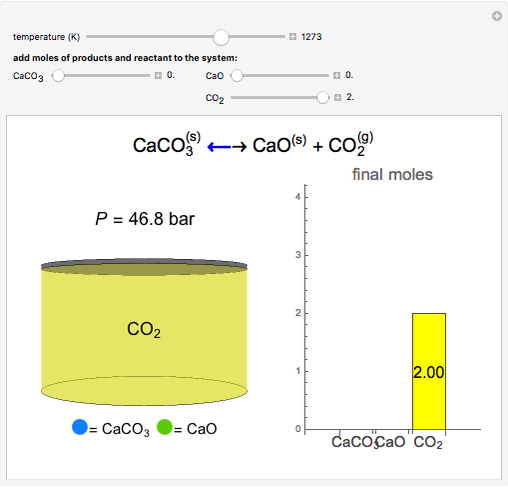
18.29 Consider the decomposition of calcium carbonate: Calculate the pressure in atm of CO 2 in an equilibrium process (a) at 25 o C and (b) at 800 o C. - ppt download

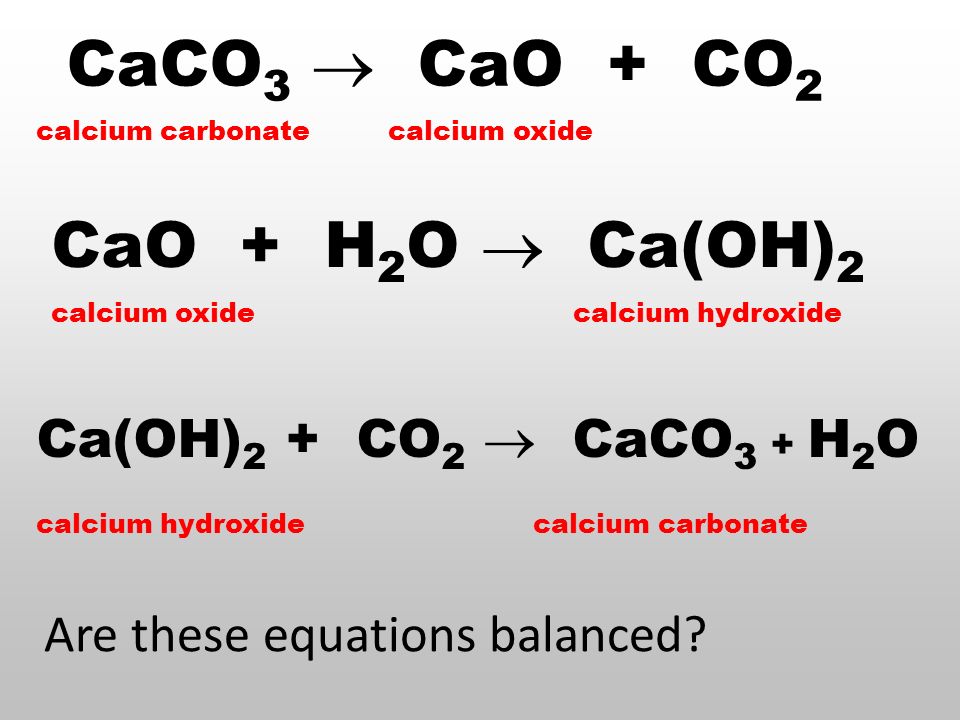
Write the balanced chemical equation of calcium carbonate decomposes on heating of lead to form - Brainly.in

Calcium carbonate decomposes, on heating , to form calcium oxide and carbon dioxide. When 10 g of - YouTube

How to Balance Ca(HCO3)2 = CaCO3 + CO2 + H2O (Decomposition of Calcium hydrogen carbonate) - YouTube