PDF) Preparation and Review of Chemistry, Manufacturing and Control (CMC) Sections of CTD Dossier for Marketing Authorization | Dhruvi Patel - Academia.edu

Control Strategy Expectations in Early Clinical Phase Synthetic Oncology Programs: Two Global Regulatory Case Studies | Organic Process Research & Development

PDF) PREPARATION AND REVIEW OF CHEMISTRY, MANUFACTURING AND CONTROL (CMC) SECTIONS OF CTD DOSSIER FOR MARKETING AUTHORIZATION

The Future of CMC Regulatory Submissions: Streamlining Activities Using Structured Content and Data Management - ScienceDirect

Transitioning Chemistry, Manufacturing, and Controls Content With a Structured Data Management Solution: Streamlining Regulatory Submissions - Journal of Pharmaceutical Sciences

CMC Considerations when a Drug Development Project is Assigned Breakthrough Therapy Status | Pharmaceutical Engineering
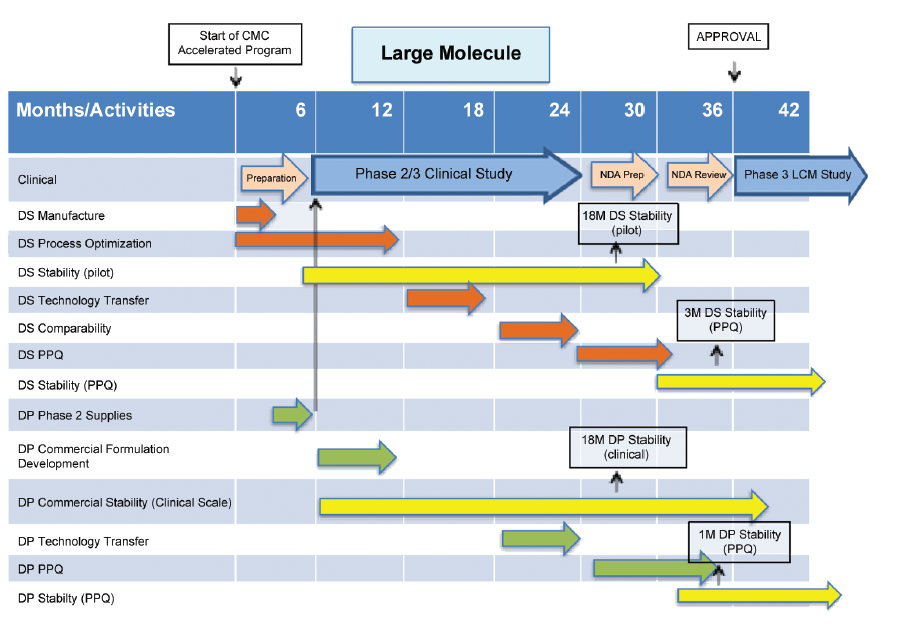
CMC Considerations when a Drug Development Project is Assigned Breakthrough Therapy Status | Pharmaceutical Engineering
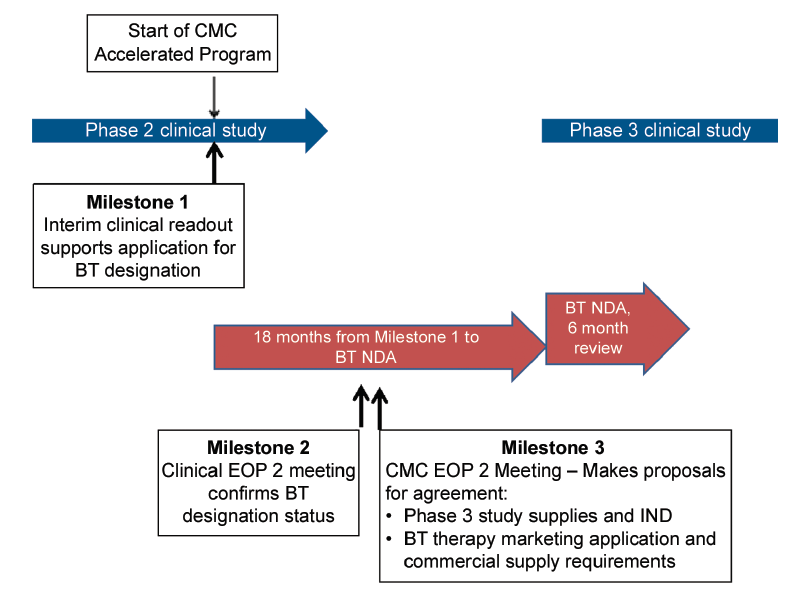
CMC Considerations when a Drug Development Project is Assigned Breakthrough Therapy Status | Pharmaceutical Engineering

The Future of CMC Regulatory Submissions: Streamlining Activities Using Structured Content and Data Management - ScienceDirect

CMC writing: Six tips for delivering top-class dossiers. - Pharmacovigilance, Auditing and Regulatory Services