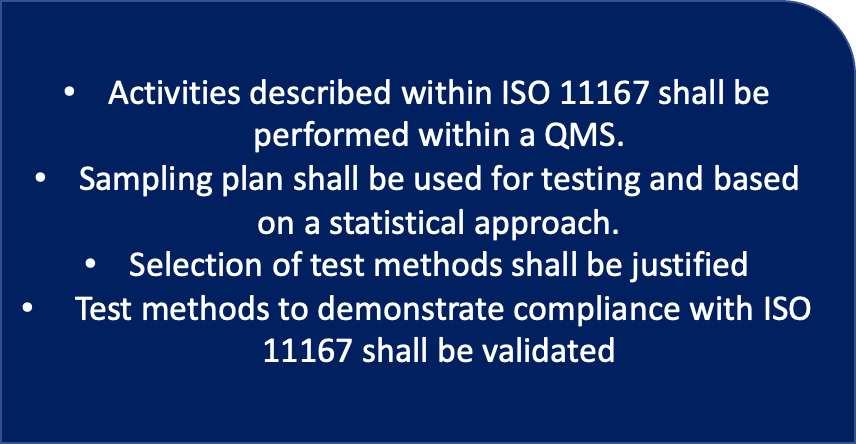
DIN EN ISO 11607-1:2014 - Packaging for terminally sterilized medical devices - Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006 + Amd 1:2014); German version EN ISO 11607-1:2009 + A1:2014

Cathriona O'Neil ISO 11607 1&2 Compliance Requirements | PDF | Verification And Validation | Sterilization (Microbiology)

Packaging Validation of Medical Devices - Impact of the Revisions of ISO 11607 & Suitable Strategies - YouTube

Packaging Validation of Medical Devices - Impact of the Revisions of ISO 11607 & Suitable Strategies - YouTube

ISO 11607-1 Packaging for Final Sterilized Medical Devices - Part 1: Rules for Materials, Sterile Barrier

EN ISO 11607-1:2020 - Packaging for terminally sterilized medical devices - Part 1: Requirements for

Learning Share Clip ISO 11607 | As of January 15, 2020, when the European Committee for Standardization (CEN) approved the 2019 ISO 11607 standards any company that markets medical... | By Adept Packaging | Facebook

EN ISO 11607-1:2009 - Packaging for terminally sterilized medical devices - Part 1: Requirements for

ISO 11607-2 Packaging for Final Sterilized Medical Devices - Part 2: Validity Requirements for Shaping, Sealing,