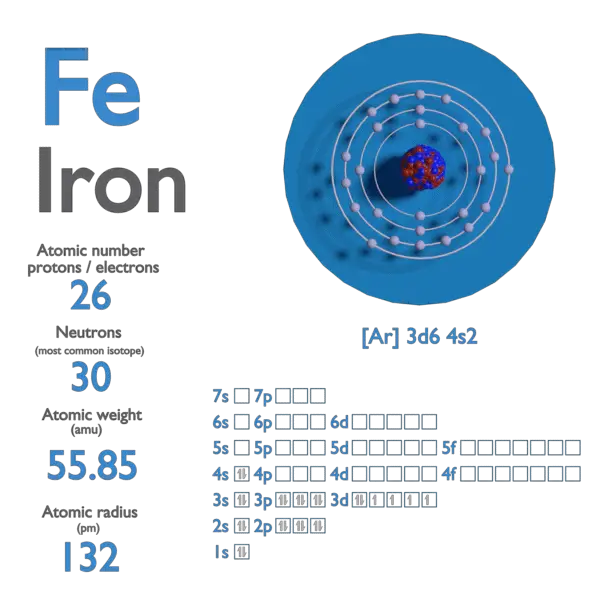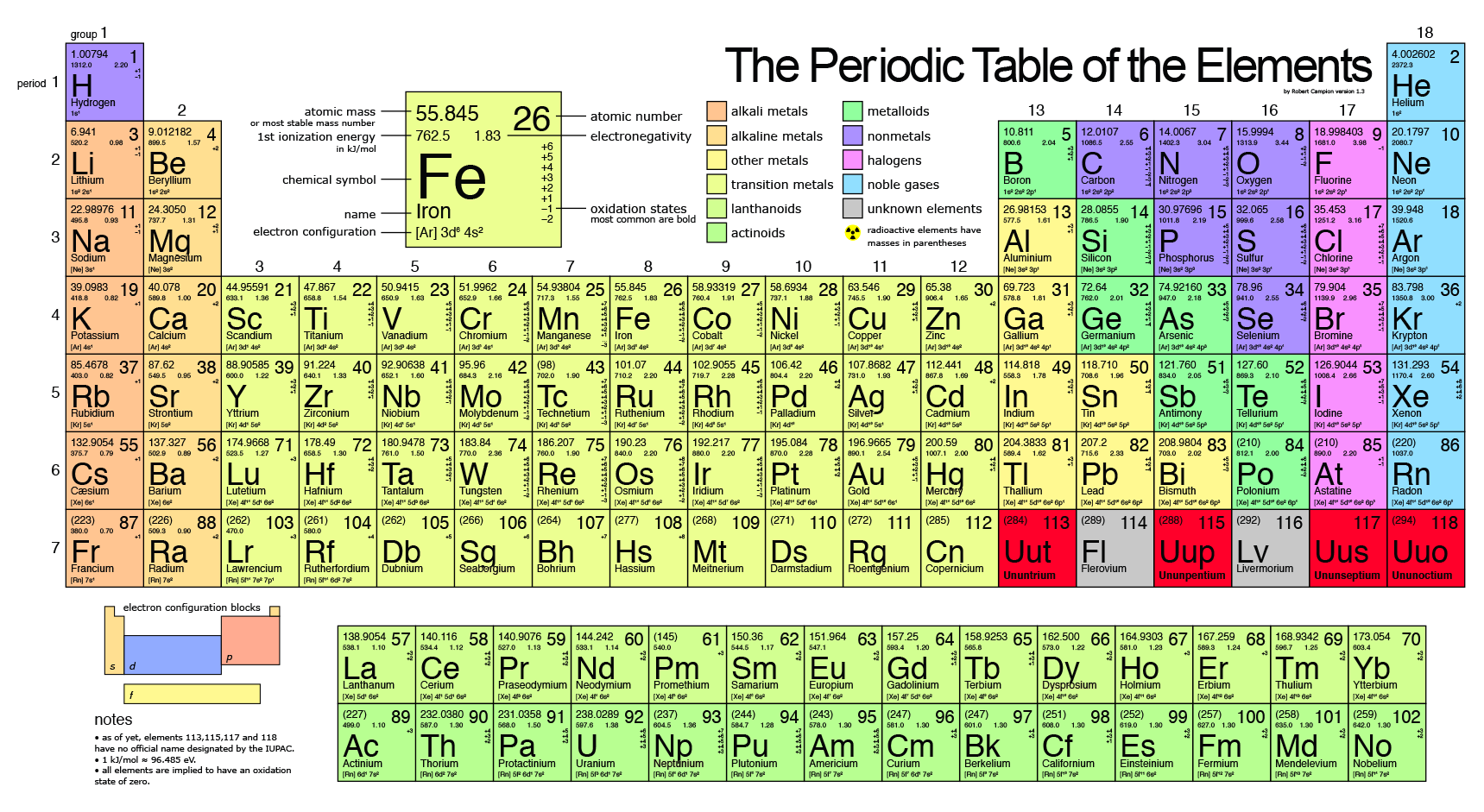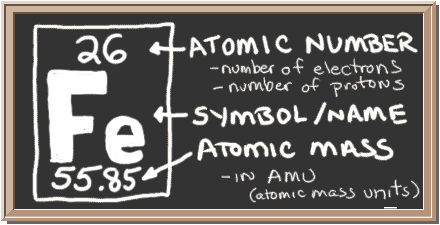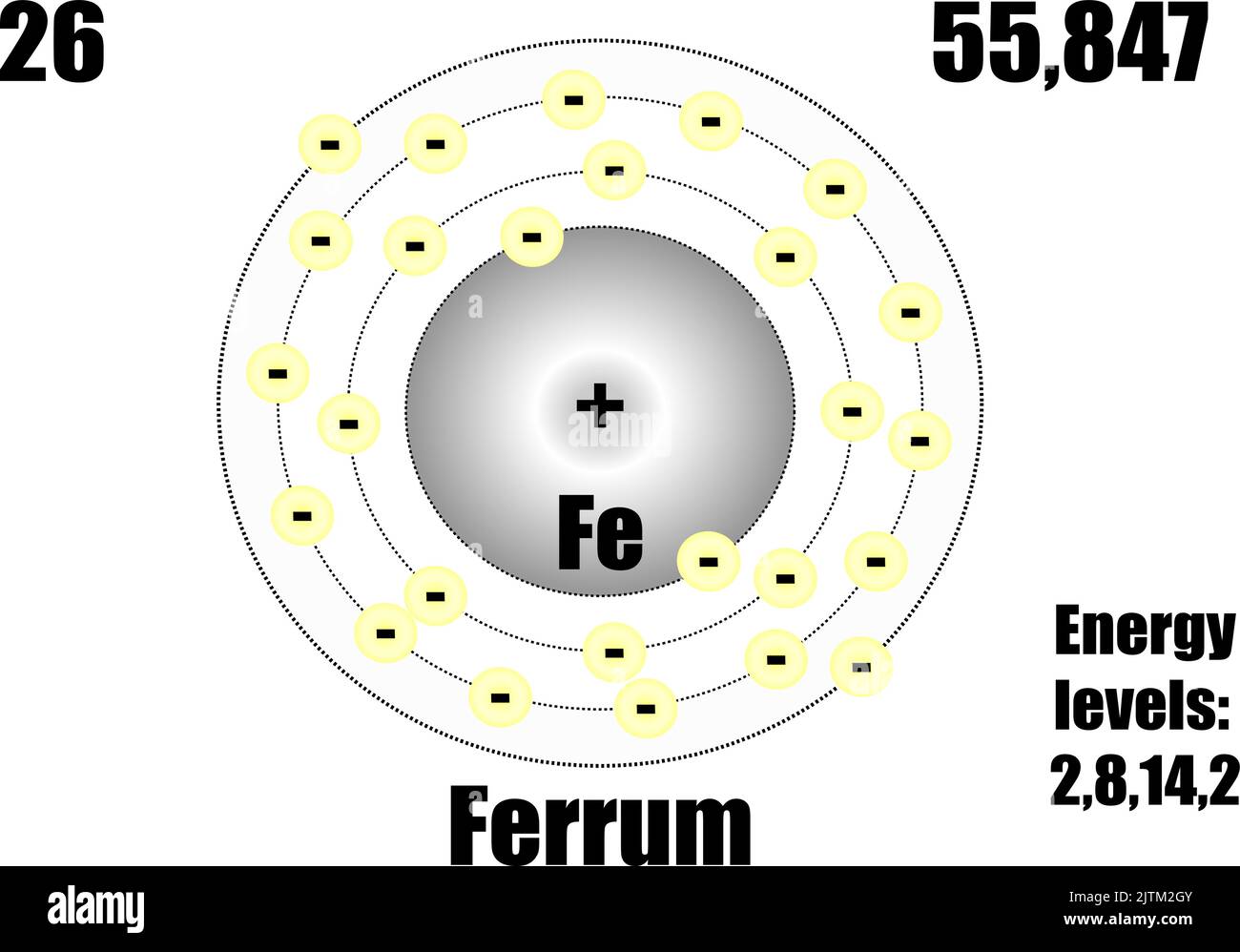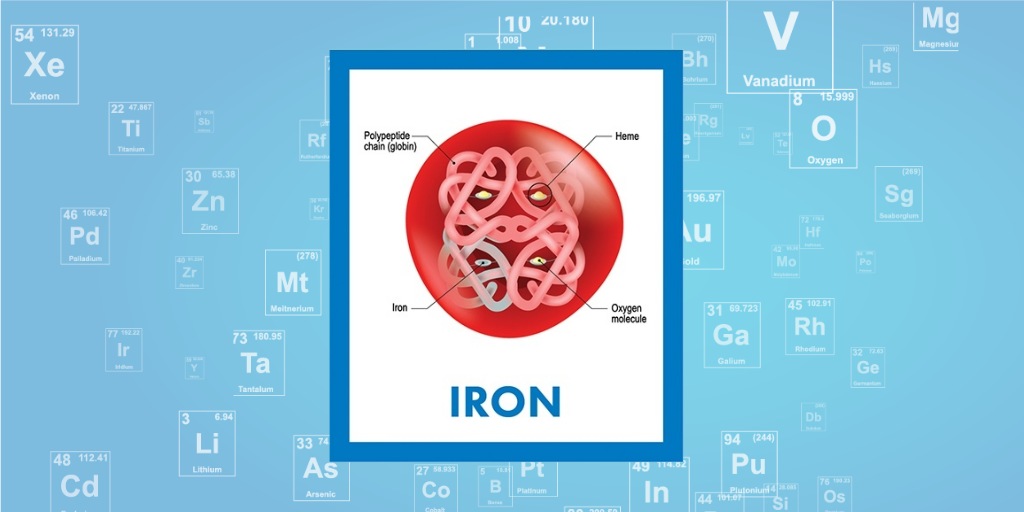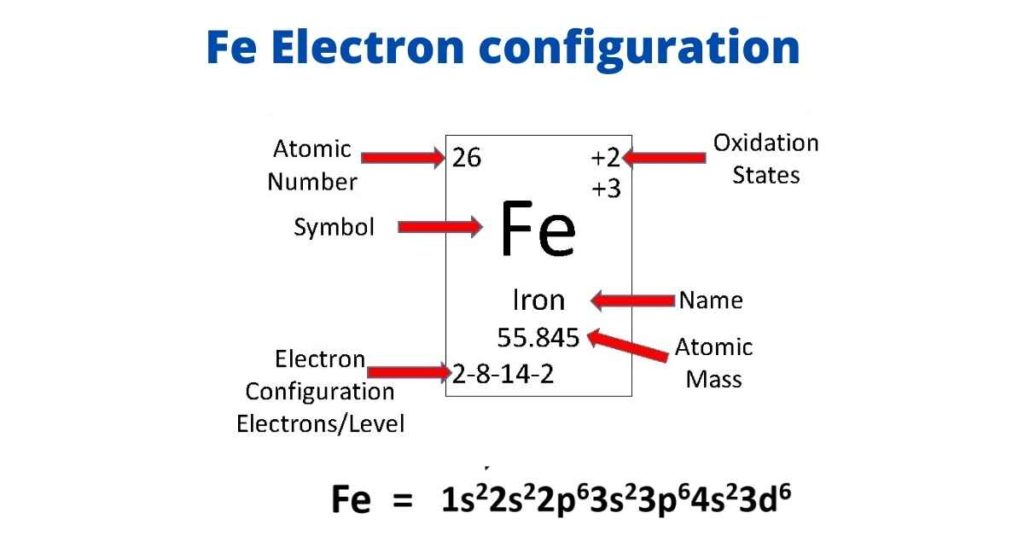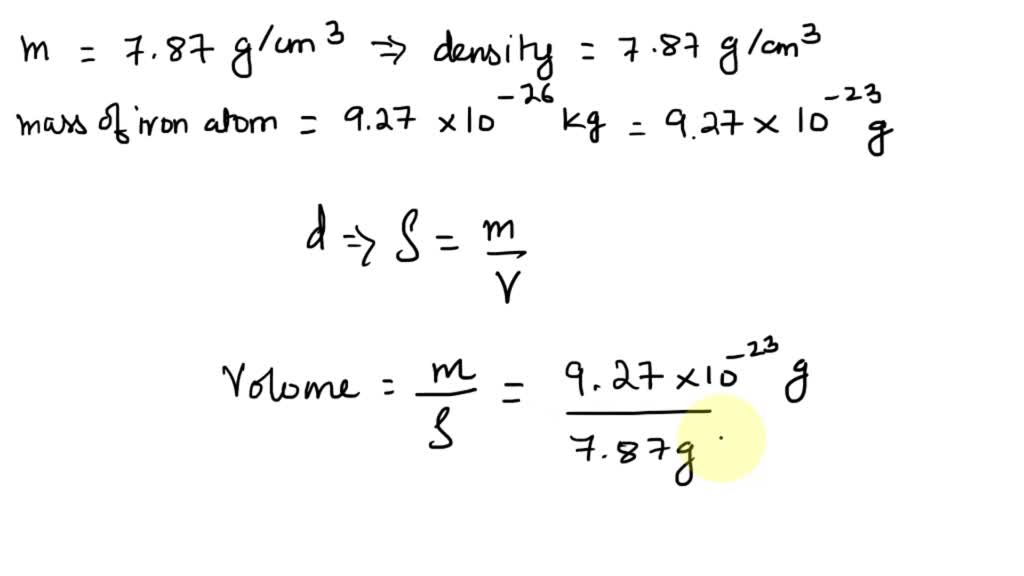
SOLVED: iron has a mass of 7.87 per cubic centimeter of a volume, and the mass of an iron atom is 9.27* 10^-26 kg. if you simplify and treat each atom as

Calculate the number of iron atoms in a piece of iron weighing `2.8 g` (Atomic mass of iron `= 56 u` - YouTube
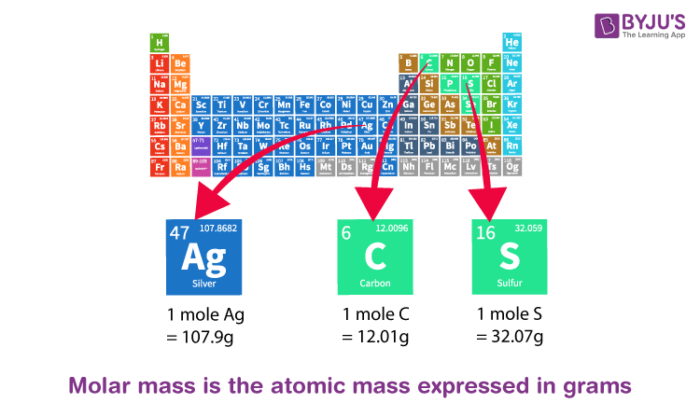
What is Atomic Mass? - List of Elements Sorted by Atomic Mass of Iron, Sulphur, Potassium, Chlorine Etc

SOLVED: 'Help! Please Hurry! Thanks! Will get brainliest for right answer. X What is true about the number 55.85 for Iron? 26 Fe iron 55.85 1. Itis the atomic number; there are

Atomic Number & Mass Number | How to Find the Atomic Mass Number - Video & Lesson Transcript | Study.com