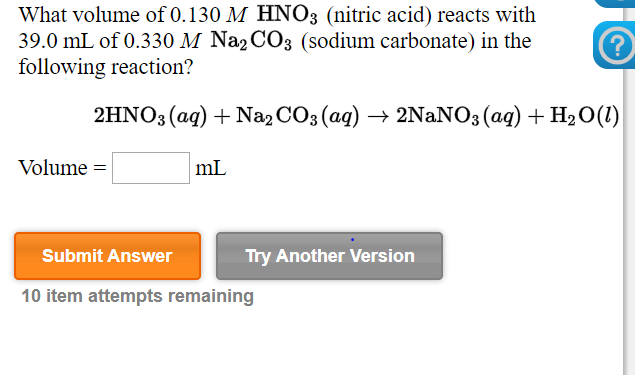SOLVED: Write net ionic equation for the reaction that occurs when zinc carbonate and excess nitric acid (aq) are combined
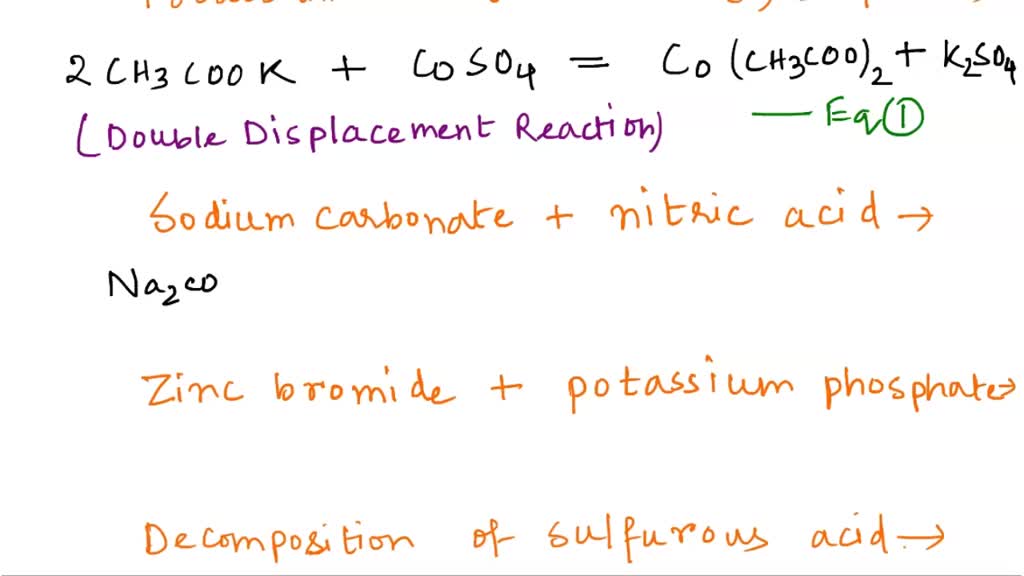
SOLVED: (h) Potassium acetate + cobalt (II) sulfate (1) Sodium carbonate + nitric acid 3 (j) Zinc bromide + potassium phosphate (k) Decomposition of sulfurous acid
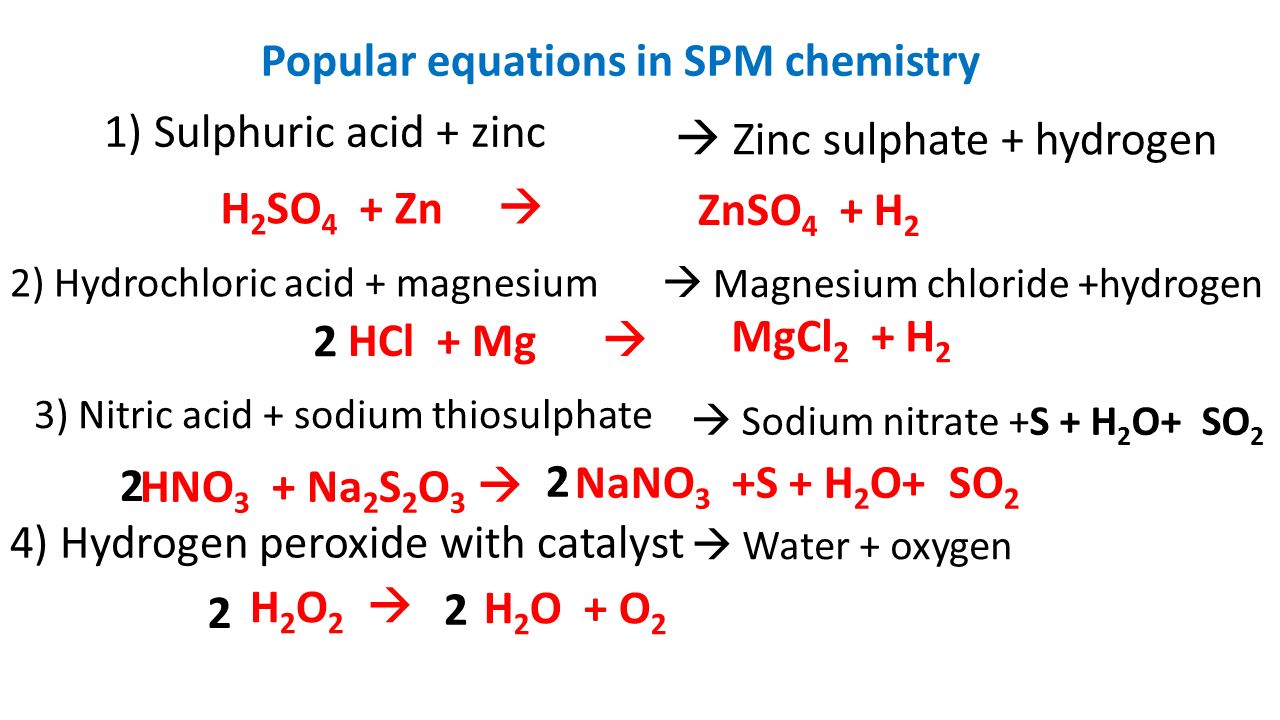
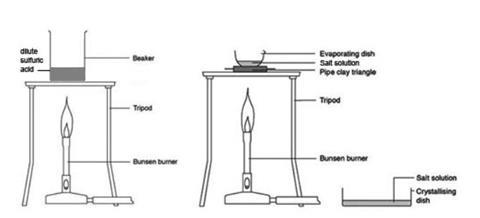
H 2 SO 4 + Zn 1) Sulphuric acid + zinc 3) Nitric acid + sodium thiosulphate 2) Hydrochloric acid + magnesium 4) Hydrogen peroxide with catalyst Popular. - ppt download

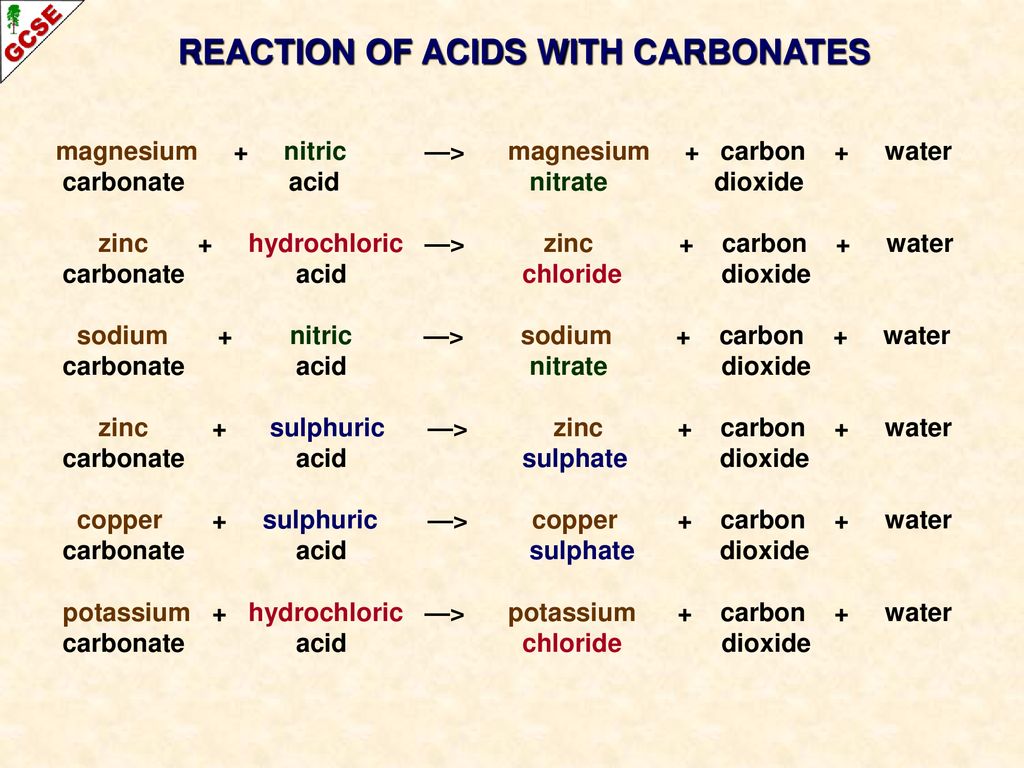
Reactions of hydrochloric sulfuric nitric acids with metals oxides hydroxides carbonates hydrogencarbonates word/symbol equations redox reaction half equations gcse chemistry revision notes igcse KS3 KS4 Science

H 2 SO 4 + Zn 1) Sulphuric acid + zinc 3) Nitric acid + sodium thiosulphate 2) Hydrochloric acid + magnesium 4) Hydrogen peroxide with catalyst Popular. - ppt download